
Please see the Transcriber’s Notes at the end of this text.
New original cover art included with this eBook is granted to the public domain.

INTERNATIONAL MEDICAL MONOGRAPHS
General Editors {
Leonard Hill, M.B., F.R.S.
William Bulloch, M.D.
THE VOLUMES ALREADY PUBLISHED OR IN
PREPARATION ARE:
THE MECHANICAL FACTORS OF DIGESTION. By Walter B. Cannon, A.M., M.D., George Higginson Professor of Physiology, Harvard University. [Ready.
SYPHILIS: FROM THE MODERN STANDPOINT. By James Macintosh, M.D., Grocers’ Research Scholar; and Paul Fildes, M.D., B.C., Assistant Bacteriologist to the London Hospital. [Ready.
BLOOD-VESSEL SURGERY AND ITS APPLICATIONS. By Charles Claude Guthrie, M.D., Ph.D., Professor of Physiology and Pharmacology, University of Pittsburgh, etc. [Ready.
CAISSON SICKNESS AND THE PHYSIOLOGY OF WORK in Compressed Air. By Leonard Hill, M.B., F.R.S., Lecturer on Physiology, London Hospital. [Ready.
LEAD POISONING AND LEAD ABSORPTION. By Thomas Legge, M.D., D.P.H., H.M. Medical Inspector of Factories, etc.; and Kenneth W. Goadby, D.P.H., Pathologist and Lecturer on Bacteriology, National Dental Hospital.
THE PROTEIN ELEMENT IN NUTRITION. By Major D. McCay, M.B., B.Ch., B.A.O., M.R.C.P., I.M.S., Professor of Physiology, Medical College, Calcutta, etc.
SHOCK: The Pathological Physiology of Some Modes of Dying. By Yandell Henderson, Ph.D., Professor of Physiology, Yale University.
THE CARRIER PROBLEM IN INFECTIOUS DISEASE. By J. C. Ledingham, D.Sc., M.B., M.A., Chief Bacteriologist, Lister Institute of Preventive Medicine, London; and J. A. Arkwright, M.A., M.D., M.R.C.P., Lister Institute of Preventive Medicine, London.
DIABETES. By J. J. MacLeod, Professor of Physiology, Western Reserve Medical College, Cleveland, U.S.A.
A Descriptive Circular of the Series will be sent free on
application to the Publishers:
LONDON: EDWARD ARNOLD
New York: Longmans, Green & Co.
INTERNATIONAL MEDICAL MONOGRAPHS
General Editors {
Leonard Hill, M.B., F.R.S.
William Bulloch, M.D.
LEAD POISONING AND
LEAD ABSORPTION
THE
SYMPTOMS, PATHOLOGY AND PREVENTION,
WITH SPECIAL REFERENCE TO THEIR
INDUSTRIAL ORIGIN AND AN ACCOUNT OF THE
PRINCIPAL PROCESSES INVOLVING RISK
BY
THOMAS M. LEGGE, M.D. Oxon., D.P.H. Cantab.
H.M. MEDICAL INSPECTOR OF FACTORIES; LECTURER ON FACTORY HYGIENE
UNIVERSITY OF MANCHESTER
AND
KENNETH W. GOADBY, M.R.C.S., D.P.H. Cantab.
PATHOLOGIST AND LECTURER ON BACTERIOLOGY, NATIONAL DENTAL HOSPITAL
APPOINTED SURGEON TO CERTAIN SMELTING AND WHITE
LEAD FACTORIES IN EAST LONDON
LONDON
EDWARD ARNOLD
NEW YORK: LONGMANS, GREEN & CO.
1912
[All rights reserved]
[v]
The Editors hope to issue in this series of International Medical Monographs contributions to the domain of the Medical Sciences on subjects of immediate interest, made by first-hand authorities who have been engaged in extending the confines of knowledge. Readers who seek to follow the rapid progress made in some new phase of investigation will find therein accurate information acquired from the consultation of the leading authorities of Europe and America, and illuminated by the researches and considered opinions of the authors.
Amidst the press and rush of modern research, and the multitude of papers published in many tongues, it is necessary to find men of proved merit and ripe experience, who will winnow the wheat from the chaff, and give us the present knowledge of their own subjects in a duly balanced, concise, and accurate form.
This volume deals with a subject of wide interest, for lead is dealt with in so many important processes of manufacture—in the making of white lead; pottery glazing; glass polishing; handling of printing type; litho-making; house, coach, and motor painting; manufacture of paints and colour; file-making; tinning of metals; harness-making; manufacture of accumulators, etc.
The authors bring forward convincing evidence, experimental and statistical, in favour of the causation of lead poisoning by the inhalation of dust. This makes prevention a comparatively[vi] simple matter, and the methods of prevention are effective, and will contribute greatly to the health of the workers and the prevention of phthisis, which is so prevalent among lead-workers. Exhaust fans and hoods, or vacuum cleaners, for carrying away the dust formed in the various processes—these are the simple means by which the dust can be removed and the workers’ health assured.
LEONARD HILL.
WILLIAM BULLOCH.
September, 1912.
[vii]
Progress in the knowledge of the use of lead, the pathology of lead poisoning, and the means of preventing or mitigating the risk from it, has been rapid of late years, and has led to much legislative action in all civilized countries. The present is a fitting time, therefore, to take stock of the general position. We have both, in different ways, been occupied with the subject for several years past, the one administratively, and the other experimentally, in addition to the practical knowledge gained by examining weekly over two hundred lead-workers.
The present treatise takes account mainly of our own persona experience, and of work done in this country, especially by members of the Factory Department of the Home Office, and certifying and appointed surgeons carrying out periodical medical examinations in lead factories. The book, however, has no official sanction.
We are familiar with the immense field of Continental literature bearing on legislation against lead poisoning, but have considered any detailed reference to this outside the scope of our book, except in regard to the medical aspects of the disease.
Most of the preventive measures mentioned are enforced under regulations or special rules applying to the various industries or under powers conferred by the Factory and Workshops Act, 1901. Occasionally, however, where, in the present state of knowledge, particular processes are not amenable to the measures ordinarily applied, we have suggested other possible lines on which the dangers may be met. We have not reprinted[viii] these regulations and special rules, as anyone consulting this book is sure to have access to them in the various works published on the Factory Acts.
The practical value of the experimental inquiry described in Chapter VI., and the light it seems to throw on much that has been difficult to understand in the causation of lead poisoning, has led us to give the results in detail.
One of us (K. W. G.) is responsible for Chapters I., III., and V. to XI., and the other (T. M. L.) for Chapters II. and XII. to XVII.; but the subject-matter in all (except Chapter VI., which is the work entirely of K. W. G.) has been worked upon by both.
Our thanks are due to the Sturtevant Engineering Co., Ltd., London; Messrs. Davidson and Co., Ltd., Belfast; the Zephyr Ventilating Co., Bristol; and Messrs. Enthoven and Sons, Ltd., Limehouse, for kindly supplying us with drawings and photographs.
September, 1912.
[ix]
| CHAPTER | PAGE | |
|---|---|---|
| I. | Historical—Chemistry of Lead | 1 |
| II. | Ætiology | 7 |
| III. | Susceptibility and Immunity | 27 |
| IV. | Statistics of Plumbism | 44 |
| V. | Pathology | 62 |
| VI. | Pathology—Continued | 81 |
| VII. | Symptomatology and Diagnosis | 110 |
| VIII. | Excretion of Lead | 127 |
| IX. | The Nervous System | 140 |
| X. | Chemical Investigations | 165 |
| XI. | Treatment | 184 |
| XII. | Preventive Measures against Lead Poisoning | 199 |
| XIII. | Preventive Measures against Lead Poisoning—Continued | 221 |
| XIV. | Preventive Measures against Lead Poisoning—Continued | 230 |
| XV. | Description of Processes | 242 |
| XVI. | Description of Processes—Continued | 265 |
| XVII. | Description of Processes—Continued | 288 |
| Index | 305 | |
| FACING PAGE |
|
|---|---|
| Plate I. | 92 |
| Plate II. | 93 |
| Plate III. | 95 |
| Plate IV. | 276 |
The use of lead for various industrial processes and for painting was well known to the ancients. Pliny[1] speaks of white lead, and a method of corroding lead in earthen pots with vinegar, sunk into a heap of dung, as the means by which white lead was made for paint. Agricola mentions three forms of lead—white lead, a compound which was probably bismuth, and metallic lead itself. The alchemists were acquainted with the metal under the name of “saturn,” the term signifying the ease with which the nobler metals, silver and gold, disappear when added to molten lead.
Colic caused by lead was also known in ancient times, and is described by Pliny; many other writers refer to it, and Hippocrates was apparently acquainted with lead colic. Not until Stockhusen[2], however, in 1656, ascribed the colic of lead-miners and smelters to the fumes given off from the molten liquid was the definite co-relation between lead and so-called “metallic colic” properly understood, and the symptoms directly traced to poisoning from the metal and its compounds. Æthius, in the early part of the sixteenth century, gave a description of a type of colic called “bellon,” frequently associated with the drinking of certain wines. Tronchin[3], in 1757, discovered that many of these wines were able to dissolve the glaze of the earthenware vessels in which they were stored, the glaze being compounded with litharge.
In our own country, John Hunter[4] describes the frequent incidence of “dry bellyache” in the garrison of Jamaica, caused by the consumption of rum which had become contaminated[2] with lead. Many other writers in ancient and historical books on medicine have written on the causation of colic, palsy, and other symptoms, following the ingestion of salts of lead; and as the compounds of lead, mainly the acetate or sugar of lead, were freely used medicinally, often in large doses, opportunities constantly occurred for observing the symptoms produced in susceptible persons. It is not to the present purpose to examine the historical side of the question of lead poisoning, but those interested will find several valuable references in Meillère’s work “Le Saturnisme”[5].
Lead was used in the seventeenth and eighteenth centuries particularly, and in the earlier part of the nineteenth, for its action upon the blood. In view of experimental evidence of the action of lead on the tissues, particularly the blood, this empirical use has interest. Salts of lead were found to be hæmostatic, and were therefore used for the treatment of ulcers because of the power, notably of lead acetate, of coagulating albuminous tissue. It was also used in the treatment of fevers, where again it is quite possible that the administration of a lead salt, such as an acetate, produced increase in the coagulability of the blood. At the same time spasms of colic and other accidents followed its use. There is practically no disease to which the human body is subject which was not treated by lead in some form or another. Lead, with the addition of arsenic, was given for malaria, while its use in phthisis was also common. The present use of diachylon plaster is an instance of the continuous use of a salt of lead medicinally, as also is the lotion of the British Pharmacopœia containing opium and lead.
—Lead belongs to the group of heavy metals, and occupies a position between bismuth and thorium in the list of the atomic weights, the atomic weight being 206·4, and density 11·85. It is blue-grey in colour, and its softness and facility to form a mark upon paper are well known. Lead melts at a temperature of 325° C., and at this temperature a certain (if negligible) amount of volatilization takes place, which vapour becomes reprecipitated in the form of an oxide. Use is made of the volatility of the metal at the higher temperatures, 550° C. and upwards, in the oxidation of lead from a mixture of lead,[3] silver, and gold; the oxide of lead, or litharge, is partially collected and absorbed by the crucible, but the greater part is mainly removed from the surface of the liquid metal as it is formed, while the richer metal is left in the crucible.
Chemically speaking lead is a tetrad, and forms a number of organic derivatives, especially through the intervention of a particular oxide, minium. Lead forms metallic alkalies and alkaline earths, resembling silver in this direction, and also metallic compounds with zinc and copper; in this point it is very similar to silver. Small quantities of lead present in other metals—as, for instance, a small trace in gold—alter its physical qualities to a great extent; whilst the addition of minute traces of other metals to lead—as, for instance, antimony—cause it to become hard, a fact made use of in the manufacture of shot.
A number of oxides of the metal are known: two varieties of protoxides (massicot and litharge), protoxide hydrate, and bioxide. Sulphide, or galena, represents the chief form in which lead is found in Nature, and from which the actual metal is produced by metallurgical processes.
The salts of lead may be divided as follows:
1. The carbonates or hydrated carbonates employed in a large number of industrial and other processes, which are the cause of much lead poisoning.
2. The acetates, both normal and basic, which are particularly concerned in the production of white lead—at any rate in the process of converting metallic lead into the hydrated carbonate through the medium of acetic acid and steam.
3. Chromate of lead, which is used as a pigment, and also in dyeing yarns, etc.
4. The nitrates and chlorides; the chloride particularly is used as an oxidizing agent (plumbing, soldering, tinning of metals).
5. The silicates, silico-borates, silico-fluoborates, which constitute the many varieties of glass and crystals used in optical instruments, and the various glazes and enamel colours used in the potteries.
There are a large number of other derivatives, but these are not of special interest to the subject in hand.
—The action of water on lead was known even to the ancients, Pliny and Galen having written on the subject. At times, and under certain conditions, as much as 20 milligrammes per litre have been found, as in the[4] Bacup epidemic, and 14 milligrammes per litre in the epidemic at Claremont. Bisserie[6] in 1900 made an exhaustive inquiry into the action of water upon lead; he gives the following conclusions:
1. Water and saline solutions attack lead more or less readily when it is in combination with another metal, such as solder, copper, bronze, iron, or nickel, the result being a hydrated oxide.
2. The maximum effect is produced with water slightly acid and with solutions of chlorides or nitrates. With these it is not necessary to have other metals present, and if the water is thoroughly aerated the pure metal is attacked.
3. Bicarbonates and carbonic acid exercise by themselves an action on wet lead, but the carbonate of lead formed in the process adheres firmly to the surface of the metal, and prevents any further action.
4. Sulphates act in the same way, but in less degree.
5. This protective action is much diminished when the water is even slightly charged with nitrates or organic material. Pouchet has pointed out that lead branch-pipes fixed to iron water-pipes, thus producing an “iron-lead couple,” set up definite electro-chemical changes, and tend to increase the rate at which solution of lead in the pipe water takes place.
Houston[7], in an extensive and very full report on the effect of water upon lead, especially undertaken for the purpose of inquiry into the contamination of supplies of drinking water by means of lead, distinguishes two species of action—namely, plumbo-solvency, which is brought about by the acidity of the water in contact with lead; and a second kind of action, erosion, determined to some extent by the dissolved air in the water. He came to the conclusion that the plumbo-solvency and erosive action of water on metallic lead differed considerably, and that the protective layer or plumbo-protective substance did not always protect lead pipes from the solvent action of water.
—A short summary of the chemistry of lead salts may not be out of place.
A soluble salt of lead, such as the acetate or nitrate, is precipitated by (1) hydrogen sulphide or alkaline sulphide as a brown or black precipitate, which is insoluble in ammonium sulphide. In dilute solutions this sulphide is, however, appreciably soluble in mineral acids, and may introduce errors in analysis, especially[5] as the solubility is distinctly increased by the presence of certain earthy salts. The sulphide produced through the action of alkaline sulphide on a soluble salt of lead is less soluble than is the corresponding acid sulphide. Soluble salts of lead are at once precipitated by albumin or peptone; the resulting precipitate has no stable composition.
Under certain conditions definite colloidal precipitates are formed, particularly in the presence of sulphide of copper or mercury. (2) Sulphuric acid or soluble sulphates produce a precipitate of lead sulphate insoluble in excess of the precipitating salt or sulphuric acid, and only slightly soluble in alkaline solutions. This method is the one generally adopted for gravimetric determination of a lead salt. (3) Potassium chromate produces a precipitate of chromate of lead very little soluble in acid, but soluble in caustic alkali. (4) Potassium iodide produces a yellow lead iodide, soluble on heating, and reprecipitating and crystallizing on cooling. (5) Alkaline chlorides and hydrochloric acid produce needle-like crystals of lead chloride soluble on heating, and reprecipitating on cooling. (6) Potassium nitrate in conjunction with a copper salt (copper acetate) produces a precipitate of a triple copper, lead, and potassium nitrate, crystallizing in characteristic violet-black cubes. This reaction is one made use of in the qualitative determination of small quantities of lead in organic fluids (see p. 167).
All the precipitates of lead salts, with the exception of the sulphide, are soluble in fixed alkalies, in ammonium acetate, ammonium tartrate, and ammonium citrate. It is possible to determine the presence of lead in a large volume without evaporating down the whole bulk of fluid. By this means liquid containing lead is treated with sulphide of copper, sulphide of mercury, or baryta-water. Meillère states that he has detected the presence of as small a quantity as 1 milligramme of lead in 1,000 c.c. of water in this manner without evaporating the liquid. Where lead is in organic combination, as is the case in the urine of persons suffering from lead poisoning, it is not decomposed by hydrogen sulphide, and the method is therefore not applicable in such cases, but is useful in water examination.
—Solutions of lead are easily electrolyzed, and give a precipitate of lead at the cathode; simultaneously the peroxide is produced at the anode, and the reaction is acid. In nitric acid solutions Riche pointed out that the whole of the lead[6] is carried to the anode, and this is the reaction made use of in the determination of lead present in the urine (see p. 172).
The presence of copper in an electrolyte regulates the precipitation of lead oxide, copper alone being deposited at the cathode, and at the same time the presence of a small quantity of copper promotes the destruction of organic materials.
[1] Pliny: lxxxiii., 11, N.c.v.
[2] Stockhusen: De Litharg. Fumo, etc. Goslar, 1656.
[3] Tronchin: De Colica Pictonum. 1758.
[4] John Hunter: Observations of Diseases of the Army in Jamaica. London, 1788.
[5] Meillère, G.: Le Saturnisme. Paris, 1903.
[6] Bisserie: Bull. Soc. Pharmacol. May, 1900.
[7] Houston: Local Government Board Annual Report, 1901-02, supplement, vol. ii.
[7]
Lead poisoning of industrial origin rarely occurs in the acute form. Practically all cases coming under the notice of either appointed surgeons, certifying surgeons, or even in the wards of general hospitals, are of the subacute or chronic type. There is no reason to suppose that lead compounds are used more frequently by the workers in lead industries as abortifacients than by other persons.
The compounds of lead which are responsible for poisoning in industrial processes are for the most part the hydrated carbonate, or white lead, and the oxides of lead, whilst a comparatively small number of cases owe their origin to compounds, such as chromates and chlorides.
The poisonous nature of any lead compound from an industrial point of view is proportional to (1) the size of the ultimate particles of the substance manufactured, and therefore the ease with which such particles are capable of dissemination in the air; and (2) the solubility of the particles in the normal fluids of the body, such as the saliva, pharyngeal and tracheal and bronchial mucus, etc., and the fluids of the stomach and intestine. An instance of the variation in size of the particles of lead compounds used industrially is the difference between ground lead silicate (fritted lead) used in the potteries, and the size of the particles of ordinary white or “raw” lead. By micrometric measurements one of us [K. W. G.[1]] found the average size of the particles of fritt to be ten times that of the white lead particles. Further, direct experiment made with equal masses of the two compounds in such a manner that the rate of settling of the dust arising could be directly compared in a beam of parallel light showed presence of dust in the white lead chamber fifteen minutes after the fritt chamber was entirely clear. It is found as a matter of[8] practice that where dust is especially created, and where it is difficult to remove such dust by exhaust fans, the greatest incidence of lead poisoning occurs. The association of dusty processes and incidence of lead poisoning is discussed in relation to the various trades in Chapters XV. to XVII. Fume and vapour given off from the molten metal or compounds, such as chlorides (tinning), are only a special case of dust.
The channels through which lead or its compounds may gain entrance to the animal body are theoretically three in number:
1. Respiratory tract.
2. Gastro-intestinal.
3. Cutaneous.
For many years most authorities have held that industrial poisoning by means of compounds of lead takes place directly through the alimentary canal, and that the poison is conveyed to the mouth mainly by unwashed hands, by food contaminated with lead dust, and by lead dust suspended in the air becoming deposited upon the mucous membrane of the mouth and pharynx, and then swallowed. As evidence that lead dust is swallowed, the classical symptom of colic in lead poisoning has been adduced, on the supposition, in the absence of any experimental proof, that the lead swallowed acted as an irritant on the gastro-intestinal canal, thus causing colic, and, on absorption from the canal, setting up other general symptoms. Much of the early treatment of lead poisoning is based upon this assumption, and the administration of sulphuric acid lemonade and the exhibition of sulphate of magnesia and other similar compounds as treatment is further evidence of the view that the poisoning was considered primarily intestinal.
One of the chief objections to this view, apart from the experimental evidence, is that in those trades where metallic lead is handled, particularly lead rolling, very few hygienic precautions have ever been taken in regard to washing before meals, smoking, etc. Although in these trades the hands become coated with a lead compound (oleate), and the workers frequently eat their food with unwashed hands, thus affording every opportunity for the ingestion of lead, the incidence of poisoning is by no means as high or so pronounced in these occupations as in those giving rise to lead dust, such as the white lead industry, where special precautions are taken, and where the incidence of poisoning is always related to the dust breathed.
[9]
—In a report on the incidence of lead poisoning in the manufacture of paints and colours, one of us [T. M. L.[2]] in 1902 laid stress on the marked incidence of poisoning in the specially dusty lead processes. Following on that report special attention was given to the removal of dust by means of exhaust ventilation. With the introduction of precautionary measures, the incidence of poisoning underwent a marked decrease, this decrease being most definite in those industries where efficient exhaust ventilation could be maintained (see p. 47). Experience shows that cases of poisoning in any given trade or manufacturing process are always referable to the operations which cause the greatest amount of dust, and where, therefore, the opportunity of inhaling lead dust is greatest.
The investigations of Duckering[3], referred to on p. 203, show the amount of dust present in the air in certain dangerous processes. His results clinch the deductions made from general observation, that dusty processes are those especially related to incidence of industrial poisoning. Ætiologically, therefore, the relationship of dust-contaminated air and poisoning is undeniable, and in not a few instances on record persons residing at a distance from a lead factory have developed poisoning, although not employed in any occupation involving contact with lead, aerial infection through dust remaining the only explanation. The actual channel through which the lead dust suspended in the air gains entrance to the body is, therefore, of especial importance; one of two channels is open—gastro-intestinal and respiratory.
The investigations of one of us (K. W. G.) on the experimental production of lead poisoning in animals has shown conclusively that the dust inhaled was far more dangerous, and produced symptoms far earlier than did the direct ingestion of a very much larger quantity of the same compound by way of the mouth and gastro-intestinal canal. There is no doubt whatever that the chief agent in causing lead poisoning is dust or fume suspended in the air. That a certain amount finds its way into the stomach direct is not denied, but from experimental evidence we consider the lung rather than the stomach to be the chief channel through which absorption takes place (see p. 81).
The following table gives a specific instance of the incidence of lead poisoning in a white lead factory, and demonstrates clearly the ætiological importance of dust. The increase in reported cases, as well as in symptoms of lead absorption not sufficiently[10] severe to prevent the individual from following his usual occupation, was associated with the rebuilding of a portion of the factory in which the packing of dry white lead had been carried on for a large number of years. The alterations necessitated the removal of several floors, all of which were thoroughly impregnated with lead dust. Before the alterations were undertaken it was recognized that considerable danger would arise; stringent precautions were therefore taken, and the hands engaged in the alterations kept under special observation. Notwithstanding this there was an increase in the number of reported cases, which were all mild cases of colic; all recovered, and were able to return to their work in a short time.
Table I.—Lead Poisoning in a White Lead Factory.
The figures refer to the weekly examination of the whole of the men. For example, if a man was returned as suffering from anæmia on three occasions, he appears as three cases in Column 7.
| Year | Total Number of Exami- nations |
Total Cases of Poisoning |
Cases in Dusty Processes |
Cases in Other Processes |
Cases of Suspen- sion |
Cases of Anæmia |
Cases of Tremor |
Blue Line | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | |||
| 1905 | 5,464 | 9 | 8 | 1 | 20 | 78 | [B] | 249 | [B] | 311 | [B] |
| 1906[A] | 5,096 | 18 | 16 | 2 | 9 | 256 | 215 | 532 | |||
| 1907 | 4,303 | 4 | 3 | 1 | 6 | 62 | 81 | 38 | |||
| 1908 | 3,965 | 4 | 3 | 1 | 5 | 40 | 25 | 11 | |||
[A] Structural alterations in progress, including cutting up “lead floor,” saturated with white lead dust.
[B] These numbers for the half-year only, the inspection being taken over in June, 1905.
Meillère[4] goes to considerable trouble to show that absorption of lead dust by the lung is hypothetical; that it may take place, but that it is not a channel of absorption of practical importance. He cites a number of opinions and experiments by various observers on the absorption of lead through the mucous membrane of the mouth, alimentary canal, conjunctiva, etc., and he regards the absorption of lead as one peculiarly confined, in the majority of instances, to the intestinal canal.
The usual view is that, in the passage of the respired dust-laden air through the nose, the larger particles of dust are deposited first of all upon the mucous membrane in the interior chambers of the nose; further, a second deposit takes place on the posterior wall of the pharynx and in the throat, where the eddies produced[11] by the current of air inhaled through the nostrils allow the finer particles to become more easily deposited. Finally, should a small trace gain access to the larynx, it is said to be deposited there upon the mucous membrane, to be subsequently ejected, and only a very small proportion of the total may ever find its way into the lung.
In all arduous labour, directly the respiration rate rises through extra calls made upon the muscles of the body, an increase in the depth of respiration takes place; yet even under these circumstances Meillère and others incline to the view that the dust is deposited on the mucous surfaces of the mouth and swallowed. Experimental evidence is entirely opposed to these suppositions. In the first place, unless particles of dust readily find their way into the lung, it is difficult to understand how the lung itself becomes the site of so much deposit of carbon, and of flinty material in stonegrinder’s pneumokoniosis. The staining of the lung by means of carbon particles, particularly in dwellers in cities, is too well known to warrant more than a passing reference. Moreover, experimental work has shown that fine powders suspended in the air easily reach the lung. Armit[5] has shown that the nickel in nickel carbonyl poisoning gains direct access to the lung, and becomes deposited there, the metallic particles being readily demonstrated in the lung tissue itself. Further, the experiments (see p. 84) demonstrate that white lead dust and other forms of lead dust definitely gain access to the lung, and thus inhaled produce all the symptoms of lead poisoning in animals subjected to the inhalation. White lead, litharge, or red lead, are not easily suspended in water, and long-continued mixing is necessary to make a suspension. Great difficulty is found in “laying” lead dust by water, as the following experiment demonstrates: Five wash-bottles are arranged in series; in the first ground dry white lead is placed, and the other three bottles are filled with water, and a tube laid under the surface of the water in such a way that the air from the first bottle must pass the whole of the water seals in each subsequent bottle. In the last bottle is dilute nitric acid saturated with sulphuretted hydrogen. If the series is now attached to an aspirating jar, and air drawn slowly over at the rate of ordinary respiration, the white lead powder in the first bottle being at the same time shaken so that the air is fully charged with finely powdered dust, lead is quickly detected in the air passing through the last bottle of the series, by[12] the darkening of the solution. In this way the presence of lead dust has been demonstrated after passing through four 2-inch water seals and 8 feet of ¹⁄₄-inch wet rubber tubing. Such an experiment negatives the theory that all, or even a large quantity, of a finely divided powder becomes deposited on the upper portion of the respiratory tract.
Particles of lead present in the air in industrial processes are exceedingly minute, and even in ground white lead the average size of the particle is under 1 μ. Finally, Tanquerel[6] and Stanski[7] succeeded in producing lead poisoning experimentally by blowing lead dust through a tube inserted in a tracheotomy opening. There remains, therefore, no room for doubt that the lung is the pre-eminent portal for lead absorption, particularly in industrial processes; from which it follows, as has been extensively shown in actual practice, that the diminution of dust in workshops and factories by means of exhaust ventilation is invariably followed by a diminution in the number of cases of plumbism.
—We have dealt with absorption by way of the lung, and have insisted that such inhalation of dust is of greater importance in giving rise to industrial lead poisoning than gastro-intestinal absorption. Gastro-intestinal absorption can take place, and is by no means negligible, in ordinary industrial conditions. One of the most interesting and important confirmatory evidences of the absorption of lead by the gastro-intestinal canal is to be found in the large outbreaks of poisoning in which water-supplies have been contaminated, either at their source or locally. We have already seen that electrolysis may play an important part in the solution of lead in water, and also learnt from Gautier[8] that the carbon dioxide content of water is not necessarily the sole predisposing element in the solution of lead. In this connection an important case is described by Thresh[9], where water by no means soft, but holding some 30 degrees of hardness, produced lead poisoning in an isolated family. The water in question was distinctly acid to litmus-paper, and contained a very high percentage of nitrates; the compound or salt of lead present was therefore one easily absorbed from the alimentary canal (see p. 86).
In all instances of water-borne lead poisoning the amount of lead present in the water was small; but as such lead would not be removed by boiling, the amount of water consumed per person from the contaminated source was probably large. As the signs[13] of poisoning did not appear until a considerable time had elapsed, a much larger quantity of lead was probably absorbed than would appear from the simple statement that the water contained ¹⁄₁₀ grain per gallon.
A number of cases have been reported from use of diachylon as an abortifacient, and the symptoms in these cases are invariably those which occur in other severe forms of poisoning such as are met with in industrial processes. In nearly every case colic was the first symptom, followed later by paresis of various types—amaurosis, albuminuria, albuminuric retinitis, melancholia, encephalopathy—and not a few of the persons succumbed. In most of the reported cases abortion was produced, but in some, particularly in one[10], three dozen pills containing diachylon were taken in a month, producing acute lead poisoning, colic, and paresis, but not abortion.
In fifteen recorded cases of the use of diachylon, fourteen showed a lead line, in many cases distinct and broad. This point has considerable interest, as such a line cannot have been produced by oral contact. The drug in the form of pills would be rapidly swallowed, and little opportunity afforded for particles to remain in the mouth. Its presence, therefore, suggests excretion from circulating blood of lead which has been absorbed in the intestine. The blue line will be referred to again later (see p. 122).
Practically all cases of water poisoning and of swallowing of lead compounds have developed colic. Further, colic is cited in all the early recorded cases, even in the very earliest cases referred to in the historical note, of lead poisoning; and as poisoning in those cases had invariably taken place by swallowing the drug, it may be presumed from this association has arisen the belief that lead must be swallowed to produce gastro-intestinal symptoms. No attention has been paid to the fact that a few cases of definite cutaneous absorption of lead from the use of hair lotions have been followed by colic. Gastro-intestinal symptoms, therefore, can be produced without the direct ingestion of the drug, and colic is a symptom of generalized blood-infection rather than a localized irritative action on the intestinal mucosa. This question, again, is more related to pathology than ætiology, and is dealt with in that section. But mention may be made here of the fact that a number of observers, more lately Meillère, have laid it down as an axiom that experimental production of lead poisoning in animals gives no criterion[14] or evidence of lead poisoning produced in man industrially. Very grave exception must be taken at once to such a statement. In the majority of experiments quoted by Meillère the quantity of lead given for experimental purposes has been large—much larger, indeed, than is necessary to produce small and characteristic effects—and instead of chronic poisoning an acute lead poisoning has generally been set up; and even where chronic poisoning has supervened, the condition has as a rule been masked by the severer initial symptoms. On the other hand, the evidence to be derived from comparison of the various observations from animal experiments brings out with remarkable unanimity the similarity of the symptoms to those produced in man, and, as will be seen later in the section devoted to Pathology, experiments by one of us (K. W. G.) have so far confirmed this surmise; in fact, a description of a case of encephalopathy coming on after lead poisoning of a chronic nature, described by Mott, agrees in practically every particular with the train of symptoms as observed in these experimental animals. Certain slight differences as to the muscles first affected are observed, but it is practically always the homologous muscle (the physiological action of which more nearly resembles the human muscle) which is the one to be affected in the animal, not the anatomical homologue. Thus, for instance, in the cat the spinal muscles, and particularly the quadriceps extensor, is the muscle which is first affected through the medium of the anterior crural nerve. This extensor muscle is one which only performs a slight amount of work in extending the knee-joint, the amount of work being, however, disproportionate to the size of the muscle. The extensors of the fore-feet ultimately do become weakened, but it is the hind-limb upon which the stress first falls.
Attention has been given to the solubility of lead salts in gastric juices, the majority of such experiments having been performed with artificial gastric juice. The method at present in use, prescribed by the amended rules of August, 1900, for earthenware and china factories, is based on some, if slight, consideration of the physiology of digestion. The method described by Rule II. states that the estimation of the quantity of lead present in the lead fritt shall be performed as follows:
A weighed quantity of dry material is to be continuously shaken for one hour at room temperature with one thousand times its weight of an aqueous solution of hydrochloric acid, containing[15] 0·25 per cent. of HCl. This solution is thereafter to be allowed to stand for one hour, and to be passed through a filter. The lead salt contained in a portion of the clear filter is then to be precipitated as lead sulphide, and weighed as lead sulphate.
This method has been adopted on the supposition that the solubility of a lead salt in the gastric juices is the chief source of the lead poisoning in the Potteries, and that the hydrochloric acid content of the solution determines, for practical purposes, the quantity of lead dissolved out of a given sample. The temperature, however, at which this estimation is made—namely, room temperature—is one considerably lower than that of the body, and the quantity of lead taken up into solution at this temperature is less than that which occurs at the ordinary temperature of the body—37° C. Practically twice as much lead is dissolved out of fritt at 37° C. for an hour as is rendered soluble at the ordinary temperature of the room—about 15° C. Thomason[11], who made some experiments in this direction, gives a figure of 2·35 lead oxide dissolved at 15° C. and 4·54 at 37° C. In another estimation—a matter, too, of some considerable importance—it was found that acetic acid dissolved 1·97 per cent, at 15° C., and 3·27 at 37° C. In lactic acid the figure was 2·28 at 15° C., and 3·53 at 37° C. It is therefore a low estimation of the solubility of any substance by the gastric juices if the substance is operated on at a temperature below that of the body.
The question of the solubility of a lead salt in the gastric contents is important in view of the small quantities of dust swallowed; and in addition to hydrochloric acid, other substances are also present in the gastric juice, which is by no means a simple aqueous solution of the mineral acid. Further, the gastric juice, except in cases of pathological type, is not acid in periods of gastric rest, unless such acidity may be represented by the presence of fermentative acids—acetic, lactic, and butyric.
The activity of the gastric juice on lead is directly caused by the quantity of organic acids present in addition to the hydrochloric acid, and by the presence of foodstuffs—(1) in the undigested and (2) in the semidigested condition. In considering the absorption of lead products from the gastro-intestinal canal, the normal digestive processes should not be lost sight of—that is, the sequence of events which occur during digestion of food. On swallowing food, no definite acidity is present in the stomach for fifteen to twenty minutes, and even after that time the[16] hydrochloric acid is only commencing to be secreted. As digestion proceeds, and the whole mass becomes partially dissolved, such portions as are in a soluble condition are passed through the pyloric opening at intervals, and the whole contents of the stomach do not pass straight through the pyloric opening as through an ordinary straight drain-pipe. As each mass of food passes onwards through the pylorus, it comes into contact in the duodenum with pancreatic juice, and with the bile, these alkaline fluids rapidly change the reaction, and allow the other ferments, trypsin, etc., to become active. As the mass proceeds onwards through the intestine, the succus entericus also exerts its function. Finally the fluid contents of the intestine are passed onwards through the ileo-cæcal valve. During the passage from the pylorus to the ileo-cæcal valve, the reaction of the intestinal contents undergoes variations, from an alkaline in the duodenum or upper parts of the jejunum, to acid at the ileo-cæcal valve. Practically no absorption takes place from the stomach itself; a small quantity of water and such highly volatile fluids as alcohol may be absorbed, but the main absorption is not commenced until the food has left the stomach; in fact, the stomach contains no mechanism for food absorption. The work of absorption of the products of digestion is carried on actively through the small intestine until finally the materials have reached the large intestine through the ileo-cæcal valve; water is then mainly absorbed, and albuminous fluids and substances in solution to some extent, but the amount of absorption which takes place is infinitesimal as compared with that of the small intestine.
These points in the physiology of digestion require to be taken into account when discussing the absorption of lead salts in the gastro-intestinal canal.
When human gastric juice is obtained direct from the stomach in man, and lead is submitted to its action, definite quantities of lead pass into solution; and, curiously enough, in the normal gastric juice lead sulphate is as soluble as both white lead and litharge. The following two tables give the results of the estimation of the direct action of human gastric juice upon lead. The particular point is that the juice was obtained by the stomach tube from persons who had been given a simple test meal preceded by a twelve hours’ fast; the juice was therefore in a normal condition. The tests gave the following results in the normal stomach:
[17]
| Lead sulphate | 0·080 | per cent. |
| White lead | 0·048 | „ |
| Litharge | 0·040 | „ |
In the second digestion, in which the analysis of the contents showed the patient to be suffering from the condition known as “hyperhydrochloridia,” the results were—
| Lead sulphate | 0·046 | per cent. |
| White lead | 0·042 | „ |
| Litharge | 0·340 | „ |
A very large number of experiments have also been performed for the purpose of determining the solubility of raw lead glaze, and white lead, in artificial digestions, the digestions having been made up in such a way that they resembled as far as possible in every particular the ordinary stomach contents. The type of digestion used was as follows:
| Dry breadcrumbs | 140 grammes. |
| Hydrochloric acid | 5 c.c. |
| Lactic acid | 0·1 c.c. |
| Acetic acid | 0·1 c.c. |
| Pepsin | 1·2 grammes. |
| Milk | 1,200 c.c. |
Digestions were performed with this mixture, and in every case the digest was divided into two portions; each portion was retained at body temperature, with agitation for a couple of hours, and at the end of that time one portion was submitted to analysis. The second portion was neutralized, sodium carbonate and pancreatic ferment added, and digestion carried on for another two and a half hours at body temperature. At the end of this time the pancreatic digest was examined.
Thirty-five digestions were performed. When 1 gramme of white lead was used—that is, 0·01 per cent., containing 0·75 per cent. of lead oxide—the quantity of lead found as lead oxide in the acid digest varied from 2 to 3 per cent., whilst the amount found in the pancreatic digest varied from 4 to 6·5 per cent. of the added salt. On increasing the amount to 12 grammes—that is, 1 per cent.—the quantity returned in the digest only increased from 1·5 to 2 per cent. In other words, in the addition of larger quantities of material the ratio of solubility did not rise in proportion to the quantity added. Where a direct pancreatic digestion was performed without the preliminary digest of the gastric contents, the amount of lead present in the digest was only about 0·2 per cent. of the quantity added; indeed, it was very much smaller than the amount dissolved out after preliminary acid digestion—that[18] is, if the normal sequence of digestion is followed, the solubility progresses after the gastric digest has been neutralized and pancreatic ferment has been added, whereas very slow action indeed occurs as the result of action of the pancreatic digest alone. Some experiments described by Thomason[12], although carried out without special regard to the physiological question of the progressive nature of digestion, distinctly confirm the point raised. Thus, in a digest of gastric juice, milk, and bread, 5·0 per cent. of lead was dissolved, whereas when pancreatic juice alone was used only 0·4 per cent. was found to be dissolved, a remarkable confirmation of the point under discussion.
The difficulty of estimating lead present in these gastric digestions is a very real one, as, owing to the precipitation of lead by various fluids of an albuminoid nature, it is difficult to determine the amount of lead present in a given quantity of digest; moreover, in making such a digest, much of the material may become entangled among the clot of the milk in a purely mechanical fashion, and, in attempting to separate the fluid from the other portion of the digest, filtration no doubt removes any lead which has been rendered soluble first of all, and reprecipitated as an albuminate. An albuminate of lead may be formed with great ease in the following way: A 5 per cent. solution of albumin in normal saline is taken, 0·02 per cent. of hydrochloric acid is added, and 10 per cent. solution of lead chloride added as long as a precipitate is formed. The precipitate is then filtered off, and washed in a dialyser with acidulated water until no further trace of lead is found in the washings. A portion of this substance taken up in distilled water forms a solution of an opalescent nature, which readily passes through the filter and gives the reaction of protein with Millon’s reagent, and the lead reaction by means of caustic potash and sulphuretted hydrogen, but very large quantities of mineral acid are required to produce any colour with hydrogen sulphide. Lead which gains access to the stomach, either dissolved in water or swallowed as fine dust, becomes in all probability converted first into a soluble substance, chloride, acetate, or lactate, which compound is then precipitated either by the mucin present in the stomach, or by the protein constituents of the food, or by the partially digested food (peptonate of lead may be formed in the same way as the albuminate described above). In this form, or as an albuminate or other organic compound, it passes the pylorus, and becomes[19] reprecipitated and redigested through the action of the pancreatic juice. A consideration of the action of artificial gastric juices and the properly combined experiments of gastric and pancreatic digestions suggest that the form in which lead becomes absorbed is not a chloride, but an organic compound first formed and gradually decomposed during the normal process of digestion, and absorbed in this manner from the intestine along with the ordinary constituents of food. Dixon Mann[13] has shown that about two-thirds of the lead administered by the mouth is discharged in the fæces, and that the remaining one-third is also slowly but only partially eliminated. This point is of very considerable importance in relation to industrial poisoning of presumably gastro-intestinal origin, and consideration of the experiments quoted suggests that the digestion of albuminate or peptonate may to some extent be the basis which determines the excretion of so much of the lead via the fæces. This alteration of solubility has no doubt a bearing on the immunity exhibited by many animals when fed with lead, and probably explains the fact that many of the experimental animals fed with lead over long periods exhibited no symptoms of poisoning (see p. 85), whereas control animals, given a far smaller quantity of lead by other means and through the lung, rapidly developed symptoms of poisoning. A diversity of opinion exists as to the effect of pepsin upon the solubility of lead. Oliver[14] considers that the pepsin has a retarding influence on the solubility of lead in the gastric juice, and Thomason’s experiments also support this view, although it is difficult to see why the action of pepsin alone should be of such extreme importance. There is also the complicating fact that other added substances in the food may mask any direct pepsin factor that may be present. Albumose and peptone rather than pepsin are to be regarded as the more important substance physiologically in their reaction with lead, and it is interesting to note that Schicksal[15] found that by exposing lead in the form of white lead in a 1 per mille solution of hydrochloric acid in the presence of peptone produced a greater solvent effect on white lead than did the diluted acid alone, and the same effect was also seen on metallic lead.
[20]
Table II.—Schicksal’s Table.
| Solution. | Substance. | Time. | Amount dissolved returned as Metallic Lead. |
|||||
|---|---|---|---|---|---|---|---|---|
| (a) | 1·0 per cent. peptone | - | 100 c.c. | White lead, 10 grms. | 3 days at 37° C. | 0·1471 | grm. | |
| 0·1 per cent. HCl | ||||||||
| (b) | 1·0 per cent. peptone | - | 100 c.c. | Metallic lead, 4 grms. | „ | 0·0330 | „ | |
| 0·1 per cent. HCl | ||||||||
| (c) | 0·1 per cent. HCl, 100 c.c. | White lead, 10 grms. | 0·0983 | „ | ||||
| (d) | 0·1 per cent. HCl, 100 c.c. | Metallic lead, 4 grms. | 0·0194 | „ | ||||
| (e) | 0·3 per cent. Na2CO3 | Metallic lead, 4 grms. | None | |||||
| (f) | 0·3 per cent. Na2CO3 | White lead | „ | |||||
| (g) | 0·3 per cent. Na2CO3 | - | White lead | „ | ||||
| 0·5 per cent. NaCl | ||||||||
| (h) | 0·3 per cent. Na2CO3 | - | Metallic lead | „ | ||||
| 0·5 per cent. NaCl | ||||||||
The experiments referred to on p. 18 undoubtedly agree with those of Schicksal. In addition to the presence of peptones, the effect of carbonic acid must be also considered, as increase in solubility in gastric and pancreatic digestions was produced when carbonic acid gas was bubbled through the digest during the period of action. The whole question of solubility of many materials in the fluids of the stomach and intestinal canal requires entire revision, not only as regards lead, but as regards a number of other metals, including arsenic.
—The final method of absorption of lead particles or lead solution into the animal body remains to be considered. Experimental phagocytosis of lead particles—as, indeed, of any minute particles of substance—suspended in an isotonic solution, may be observed directly under the microscope. Lead particles show no exception to the rule, and white blood-corpuscles in a hanging-drop preparation, made by suspending them in an isotonic salt solution and serum, may be watched englobing particles of lead, and by appropriate means the ingested lead may be afterwards demonstrated. In such an experiment, much of the lead absorbed by the individual corpuscles rapidly loses its property of giving a black precipitate with sulphuretted hydrogen, and has apparently become converted into an organic compound, peptonate or albuminate.
In the section devoted to the Chemistry of Lead, it has been[21] noted that the colloidal solutions of lead are not precipitated by sulphuretted hydrogen, and that albuminates and peptonates of lead are presumably of colloidal form. There seems evidence, therefore, that the direct absorption of lead takes place by means of the phagocytes of the body, and that in them it becomes converted into a colloidal form, in which it is probably eliminated through the kidney and intestine, mainly the latter.
Further evidence of the englobement of lead particles by amœbic cells may be gained if sections of the intestines of experimental animals are examined; in the lymphoid glands particles of lead may be seen situated in the interior of the walls, and even in the cells. It does not by any means follow that these particles of lead sulphide present in the cells have been formed in situ; more probably the lead has been converted into a sulphide in the intestinal lumen itself, and subsequently taken up by the amœbic cells situated in its periphery.
Another solution is possible—namely, that the particles seen in the intestinal wall are particles of lead in process of excretion into the intestine itself, and that the pigmentation of the vessel walls and cells is caused by the staining of the particles of lead passing from the blood into the lumen of the tube, which have been converted into a sulphide during their passage.
The localization of the staining in the large intestine, especially in the region of the appendix in animals (cats), tends to support this theory. The large bowel near the ileo-cæcal valve, the appendix, and even the glands in the immediate neighbourhood, are found to be discoloured, and to contain lead in larger quantities than any other portion of the intestine. In extreme cases the whole of the large intestine may be stained a greyish-blue. The bloodvessels in the mesentery in this region are also engorged. When, however, a salt of lead, such as lead carbonate or lead oxide, gains access to the stomach, it may be easily converted into chloride by the free hydrochloric acid present in the stomach; and, in addition, should there be any chronic acid-dyspepsia (hyperchlorhydria), particularly of the fermentative type, in which free lactic acid and other organic acids are to be found within the viscus, small quantities of lead swallowed as dust undergo solution and conversion into chloride or lactate. The pouring out of acid gastric juice from the stomach glands does not take place immediately after the first bolus of food is swallowed, and it may be twenty minutes or half an hour before[22] the gastric contents have an acid reaction. During this time any lead salts previously swallowed may become incorporated with the bolus of food and escape absorption.
Lead in solution or suspension in the stomach which becomes mixed up with the food, and at the same time subjected to the action of various albuminous constituents of the food in addition to acids, causes an albuminate or peptonate of lead to be easily formed, and as such can never be absorbed from the stomach direct; practically no absorption takes place in the stomach, and the presence of food containing albuminate precipitates any lead in solution as an organic insoluble salt. The bolus of food impregnated with small quantities of lead passes onwards to the intestine, where further digestion takes place. As the mass passes through the intestine the action gradually results in the reappearance of acidity, but at the same time a certain quantity of sulphuretted hydrogen is produced, some of it from the degradation of the sulphur-containing moiety of the protein molecule by ordinary hydrolytic process and intestinal ferments, quite apart from any bacterial action. A portion of the lead present in the chyme may be set free again for absorption. The bile is said to assist in the solution of lead in vitro.
In experiments made by one of us, which are quoted later, it has been shown that an isolated loop of intestine allows the absorption of a soluble lead salt (chloride) when there is no food present in the loop. As the food mass proceeds through the length of the intestine more and more sulphur is set free, and an opportunity arises for the fixation of the lead as a sulphide, but even as a sulphide it is slightly soluble. Probably, however, most of the lead becomes absorbed long before it reaches the stage at which free sulphur or sulphuretted hydrogen exists for the formation of sulphide. It is highly probable that lead, in common with a number of other heavy metals, including arsenic, is absorbed gradually in the upper part of the intestine, and re-excreted in the lower. Such an hypothesis is undoubtedly strongly supported by the remarkable staining of the large intestine and the ileo-cæcal valve.
The exact mechanism of the absorption of lead from its compound with albumin or peptone as a lead peptonate or albuminate is very difficult to state at present; lead albuminate is undoubtedly insoluble in water or normal saline and in albumin. The process of absorption, then, of the metal lead from the gastro-intestinal[23] canal is very closely related to the absorption of other heavy metals, and the fact that animals after very large doses of lead salts administered via the mouth show hæmorrhages in the intestinal wall, in addition to hæmorrhages in other parts of the body, with occasional distinct ulceration, suggests a localized coagulative action on the vessels in the wall of the intestine as the probable origin of the ulceration. A consideration of this problem of lead absorption from the intestine—probably only the minutest quantity of lead, if any, is absorbed from the stomach direct—is one of considerable importance in the prevention of such lead poisoning as is attributable to swallowing lead. No work in a lead factory should be commenced in the morning without partaking of food, because if food be present the opportunities for absorption of lead are greatly diminished, and of all foods the one to be recommended as the most efficient is milk, or cocoa made with milk.
The absorption of dust through the lung is probably an exceedingly complicated reaction, and Armit’s experiments with nickel carbonyl probably give the clue. He found that in nickel carbonyl poisoning the volatile product was split up on the surface of the lung cells, the metallic portion passing onwards into the lung itself, to be eventually absorbed by the serum.
From the pathological and histological investigations described on p. 81, and from the fact that particles of lead are very readily taken up by white blood-corpuscles, we can conclude that absorption of the finer lead particles gaining access to the lung takes place through the medium of these phagocyte cells, as such cells are well known to exist within the alveoli of the lung. The stored-up carbon particles found in the lungs in dwellers in cities show that such transference of particles from the alveoli to the inner portions of the lung trabeculæ is a constant phenomenon, and it is therefore easily understood how rapidly any fine particles not of themselves irritant may be easily taken up by the tissues. Once having gained access to the interior of the cells, the particles subjected to the action of the serum of the blood in the ordinary process of bathing the tissues by the exuding lymph—nay, more, actual particles of lead—may thus be actually transferred bodily into the finer blood-spaces, and so be carried forward to the general circulation. Such particles as remain fixed in the lung will undergo gradual absorption, and the constant presence of carbonic acid in the circulating blood brought to the[24] lung undoubtedly largely contributes to their solution, and there is no need to presuppose the necessity of some recondite interaction of organic acid for the solution of the inhaled lead in the lungs.
In the absorption of the substance from the intestine, it may go direct into the blood-stream in a similar fashion through the lacteals along the lymph channels, and so into the thoracic duct, and finally into the general circulation. On the other hand, a certain amount, probably not an inconsiderable portion, is taken up by the portal circulation and transferred direct to the liver itself. Chemical analysis of the liver supports this view, as does also the considerable amount of stress thrown upon the liver when poisoning has taken place from the intestinal canal on administration of massive doses of a highly soluble lead compound. According to Steinberg[16], excretion of lead takes place partly from the liver by the bile. This is probable, but there is no experimental evidence at the present time to support the view. If such an excretion does take place, the form in which the lead is excreted is probably one in which it is no longer soluble by digestive action. On the other hand, it may be in so soluble a form as to become reabsorbed from the intestine, thus setting up a constant cycle. But such a theory is one that would require a considerable amount of experimental evidence to support it before it could be relied on.
There is no doubt that, however absorbed, lead remains stored up in the body in minute quantities in many places, and the close analogy to arsenic is met with in the curious elimination of the metal by the fæces. Cloetta[17], quoted by Dixon Mann, discovered that, although dogs were unable to take a larger dose of arsenic than 0·0035 gramme per day without exhibiting toxic results, they could nevertheless take arsenic in much larger doses if it were given in the solid form, and he was able to increase the dose to as much as 2 grammes per diem without showing any toxic symptoms. Examination of the urine and fæces showed that as the amount of urinary excretion of arsenic diminished, so that in the fæces increased, and in lead poisoning, even in massive doses swallowed in error, the amount of lead excreted by the urine rapidly diminishes in quantity, although the patient may be still suffering from the effects of lead poisoning. The experiments, also, quoted on p. 100 constantly pointed to the elimination of lead by way of the intestine,[25] and in practically all the animals that had suffered from chronic poisoning well-marked dark staining of the upper part of the cæcum due to lead was invariably present. This staining and excretion of lead of the large intestine undoubtedly takes place in man. In a case described by Little[18], where diachylon had been administered, the administration of a large enema containing sulphate of magnesium came away black. A more detailed result of the experiments and a consideration of the elimination of lead are reserved for another chapter, but it is impossible to consider the ætiology of the disease without some reference to the general histological channels of absorption and excretion.
—A considerable amount of controversy has centred on the question of the absorption of lead through the unbroken skin. It has been shown that such drugs as belladonna applied to the skin alone may produce dilatation of the pupil; an ointment containing salicylic acid spread upon the skin and thoroughly rubbed in is followed by the appearance of derivatives of salicylic acid in the urine; mercury may be applied to the skin, and rubbed in, in sufficient quantities to produce salivation; and a very large number of other drugs may be cited, all of which when applied to the unbroken epidermis with friction produce the physiological action of the drug.
There is no reason to exclude lead from the category of drugs which may be absorbed through the medium of the skin, and, as several observers have shown, animals may be poisoned by lead on applying a plaster of lead acetate to the skin. Amongst these experiments may be quoted those of Canuet[19] and Drouet[20] on rabbits. Some observers, among whom may be mentioned Manouvrier[21], have attempted to prove that paralysis of the hands occurs more often in the right hand in right-handed people, in the left hand with left-handed people, and from the various experiments showing absorption of lead through the unbroken skin they seek to connect the lesion of the nerve with absorption direct through the skin of the hands.
Many objections can be urged against acceptance of this theory. Lead workers who are constantly manipulating lead in a state of solution with bare hands do not appear as a class to be more subject to wrist-drop than do persons who are exposed to inhalation of fumes or dust of lead; in fact, incidence of paralysis and of nerve lesions generally is more severe among persons[26] exposed to prolonged inhalation of minute quantities of lead through the respiratory tract. The greater the exposure to dust, the greater the number of cases of anæmia and colic, whilst in other industries, as has already been stated, where lead exists as an oleate on the hands of the workers day in and day out for many years, paralysis and even colic are of rare occurrence; in other words, persons especially exposed to the absorption of lead through their hands show a much smaller incidence of lead poisoning of all types than do those exposed to lead dust. Further, the pathology of wrist-drop and similar forms of paresis tends to show that the nerve supplying the affected muscles is not affected primarily, but that the initial cause is hæmorrhage into the sheath of the nerve, producing ultimate degenerative change. The hæmorrhage, however, is the primary lesion.
[1] Goadby, K. W.: A Note on Experimental Lead Poisoning. Journal of Hygiene, vol. ix., No. 1, April, 1909.
[2] Legge, T. M.: Report on the Manufacture of Paints and Colours containing Lead (Cd. 2466). 1905.
[3] Duckering, G. E.: Journal of Hygiene, vol. viii., No. 4, September.
[4] Meillère, G.: Le Saturnisme, chap. iv. Paris, 1903.
[5] Armit, H. W.: Journal of Hygiene, vol. viii., No. 5, November, 1908.
[6] Tanquerel des Planches: Traité des Maladies de Plomb, ou Saturnines. Paris, 1839.
[7] Stanski: Loc. cit.
[8] Gautier: Intoxication Saturnine, etc. Académie de Médecine, viii., November, 1883.
[9] Thresh, J. C.: The Lancet, p. 1033, October 7, 1905.
[10] Ibid., January 5, 1909.
[11] Thomason: Report of the Departmental Committee on Lead Manufacture: Earthenware, China, vol. ii., appendices, p. 61. 1910.
[12] Ibid.
[13] Dixon Mann: Forensic Medicine and Toxicology, p. 495. 1908.
[14] Oliver, Sir T.: Lead Poisoning (Goulstonian lectures). 1891.
[15] Schicksal: Die Bekämpfung der Bleigefahr in der Industrie, p. 38. 1908.
[16] Steinberg: International Congress of Industrial Hygiene. Brussels, 1910.
[17] Cloetta: Dixon Mann’s Forensic Medicine and Toxicology, p. 463.
[18] Little: The Lancet, March 3, 1906.
[19] Canuet, T.: Thèse, Paris, 1825, No. 202. Essai sur le Plomb.
[20] Drouet: Thèse, Paris, 1875. Recherches Experimentales sur le Rôle de l’Absorption Cutanée dans la Paralysie Saturnine.
[21] Manouvrier, A.: Thèse, Paris, 1873, No. 471. Intoxication par Absorption Cutanée.
[27]
A large number of poisonous substances, among which lead may be included, are not equally poisonous in the same dose for all persons. It is customary to speak of those persons who show a diminished resistance, or whose tissues show little power of resisting the poisonous effects of such substances, as susceptible. On the other hand, it is possible, but not scientifically correct, to speak of immunity to such poisonous substances. Persons, particularly, who resist lead poisoning to a greater degree than their fellows are better spoken of as tolerant of the poisonous effects than as being partially immune.
The degree of resistance exhibited by any given population towards the poisonous influence of lead shows considerable variation. Thus, in a community using a water-supply contaminated with lead, only a small proportion of the persons drinking the water becomes poisoned. There are, of course, other factors than that of individual idiosyncrasy which may determine the effect of the poison, as, for example, the drawing of the water first thing in the morning which has been standing in a particular pipe. But even if all disturbing factors are eliminated in water-borne lead poisoning, differing degrees of susceptibility are always to be observed among the persons using the water.
Lead does not differ, therefore, from any other drugs to which persons show marked idiosyncrasies. Thus, very small doses of arsenic may produce symptoms of colic in susceptible persons; a limited number of individuals are highly susceptible to some drugs, such as cannabis indica, while others are able to ingest large doses without exhibiting any sign of poisoning; and it is well known that even in susceptible persons the quantity of a particular drug which first produces symptoms of poisoning may[28] be gradually increased, if the dosage be continued over long periods in quantities insufficient to produce marked symptoms of poisoning. In this direction a number of experiments have been performed with arsenic, particularly those of Cloetta[1], who found that the dose of arsenic for dogs could be gradually raised, if given by the mouth, to many times the ordinary fatal dose, but that if at this point a subminimal fatal dose was injected beneath the skin acute symptoms of arsenic poisoning followed.
We show in a later chapter that the excretion of lead in persons tolerant of the metal takes place through the medium of the bowel, and that probably those individuals who are engaged in what are recognized as dangerous processes in lead industries, and yet show no signs of illness, have established a kind of balance between the intake of the poison and its excretion by the bowel. It is rarely possible in such persons to find any lead excreted through the kidney. Occasionally, however, such persons, after working a considerable time in a dangerous lead process, become suddenly poisoned, and inquiry frequently discloses the fact that some disturbing factor, either intercurrent illness, alcoholic excess, etc., has occurred, or that the breathing of a big dose of dust has precipitated the symptoms of general lead poisoning. On the other hand, the experience of all persons engaged in the routine examination of lead workers is that, although a worker may show signs of lead absorption as distinguished from definite lead poisoning during the earlier period of his employment, he later shows less and less signs of the influence of the poisonous substance; even a mild degree of definite poisoning in the early stages of work in a lead process does not seriously militate against this gradually acquired tolerance, whilst careful treatment during such a time as the man is acquiring tolerance to the poison frequently tides him over the period, and enables him to withstand the ordinary dangers attached to his work.
The earliest symptom of lead absorption is anæmia. The anæmia is not very profound, and the diminution in the red blood-cells rarely reaches as low as 2,000,000 per c.c., the hæmoglobin remaining somewhere between 75 and 80 per cent. Some loss of orbital fat, as well as fat in the other parts of the body, occurs, but beyond this no obvious clinical signs of poisoning exist. Should such persons possess unhealthy gums, a blue line rapidly makes its appearance, but where the gums are healthy it is unusual to see any sign of deposit in this prodromal stage.
[29]
Persons who gradually acquire tolerance go through the stage of anæmia without exhibiting any symptoms of colic or paresis, and without any treatment the hæmoglobin and the number of red cells gradually pass back to a more or less normal condition. During this period—that is, whilst the blood shows signs of a diminution in its corpuscular and colour content—basophile granules may always be found if sought for, but disappear as a rule when the blood-count has returned to about 4,000,000 per c.c. and an 80 per cent. hæmoglobin. Such a man has now developed tolerance to the poisonous influence of lead, a tolerance which may be described as a partial immunity produced by recurrent subminimal toxic doses. On the other hand, in a number of persons who show definite susceptibility, the blood-changes are progressive, and do not show signs of automatic regeneration. In such persons, even after so short time as four to six weeks’ exposure to lead absorption, definite symptoms of colic may make their appearance. The removal of such an individual from the poisonous influence of lead generally clears up the symptoms in a short time, but the symptoms may occasionally continue for several months after removal from the influence of the poison. An individual of this type is to be looked upon as showing peculiar susceptibility, and should not be employed in any lead process where there is risk.
Such statistics as are available on this point show that an increased tolerance to the poisonous influence of lead is gradually acquired during periods of work, in that the number of attacks of poisoning diminish in frequency very considerably in relation to the number of years worked. As will be seen on reference to the chapter dealing with the statistics of lead poisoning (p. 46), the greatest number of cases occur in persons who have only worked a short time in lead. On the other hand, the sequelæ of lead poisoning only make their appearance, as a rule, after long-continued exposure. It is important to bear in mind that the various forms of paresis rarely make their appearance unless the subject has been exposed to long-continued absorption of lead, and, further, that the blood of such persons will as a rule show, on careful examination, evidences of the long-continued intoxication. If measures, therefore, were taken to determine the presence of such continued intoxication, and to diminish the amount of poison absorbed (subjecting the individual at the same time to a proper course of treatment), a large number of the cases of[30] paralysis, encephalopathy, and death, incidental to the handling and manufacture of lead, could be eliminated.
Susceptibility may at times be shown by several members of one family. Oliver[2] says that he has known many members of one family suffer from and die of lead poisoning. In our experience several instances of this susceptibility have been noticed. In one case two brothers, working in one shift of men, developed poisoning, although no other persons in that shift showed any signs of it. A third brother, who came into the works after the other two had left, and who was placed under special supervision on account of the susceptibility exhibited by his two brothers, although given work which exposed him to the minimal degree of lead absorption, developed signs of poisoning six weeks after his entrance into the factory. In another factory, three sons, two daughters, and the father, all suffered from lead poisoning within a period of four years: the father had three attacks of colic, ultimately wrist-drop in both hands; one daughter had one attack of colic, and the other three attacks; whilst the three brothers all suffered from colic and anæmia, and one had early signs of weakness of the wrist. There was no evidence at all to show that these persons were more careless, or had been more exposed to lead dust, than any other of the persons with whom they worked, or that the work they were engaged upon was more likely to have caused illness to them than to other workers. Persons with a fresh complexion and red hair have been noted to be more susceptible to lead poisoning than dark-haired persons.
In one factory with which we are familiar, a number of Italian workmen are employed; these show considerably less susceptibility to lead poisoning than do their English comrades as long as they adhere to their own national diet. When, however, they give this up, and particularly if they become addicted to alcohol, they rapidly show diminished resistance; in fact, all the cases of plumbism occurring among the Italians in this factory during the last ten years have been complicated with alcohol. It is possible that the relatively large quantity of vegetables in the diet of these Italians influences the elimination of absorbed lead. There is some reason to suppose, however, that there may be racial immunity to lead poisoning.
The following case in the same factory illustrates a point already mentioned—namely, the gradually acquired tolerance to poisoning,[31] and the unstable equilibrium existing. The individual was a man of twenty years of age. He commenced work on August 2, 1905. Six weeks later he was under treatment for seven weeks, for lead absorption, and had a peculiarly deep blue line round his gums, and a diminished hæmoglobin of 75 per cent. The symptoms disappeared with ordinary routine treatment, and his work was shifted to a position in the factory where he was exposed to the minimum amount of lead absorption, at which work he continued during the rest of the time he remained in the factory. He continued quite well until June, 1906, when he was again under treatment for two weeks, with the same blue line and anæmia, and his blood showed the presence of basophile granules. He was under treatment again in January and February, 1909, for five weeks, had again a deep blue line and basophile staining of his blood. On November 7, 1911, having had no anæmia and no blue line, he had a slight attack of colic. During this period of work his blood had been examined on eight occasions, and on each occasion it had shown basophile granules. The attack of colic was an exceedingly mild one. There is no reason to suppose that he had indulged in alcoholic excess, but there was some reason to think that for about a month he had been subjected to increased lung absorption. No other persons working in the same shift at the same work developed poisoning during the whole of this period. This case illustrates initial susceptibility, partial tolerance, and ultimate breaking down of such partially established tolerance.
During the experimental inquiry on lead poisoning by one of us [K. W. G.[3]], the question of the subminimal toxic dose and the minimal toxic dose was under consideration. Animals subjected to inhalation of lead dust invariably succumbed to the effects of the poison when the dose given represented from 0·0001 to 0·0003 gramme per litre of air inhaled, the period of inhalation being half an hour three times a week. On the other hand, when the lead content of the air was as low as 0·00001 gramme per litre, the symptoms of poisoning were long delayed, and in more than one instance, after an early diminution in weight, recovery of the lost weight took place, and the animals, whilst showing apparent symptoms of absorption, had no definite symptoms of paresis. These observations tend to confirm such clinically observed facts as are given in the case cited above, but they, of course, do not form a criterion as to the[32] amount of lead dust which may be regarded as innocuous to man.
Lead is peculiarly a cumulative poison, and post-mortem analyses of viscera show that it may be stored up in certain parts of the body, more especially in the bone and red bone marrow and brain, and to some extent in the liver, spleen, and kidneys. Any circumstance, therefore, that temporarily interferes with the ordinary channels through which lead is excreted may determine the presence of a much larger quantity than usual of the metal in circulation in the body; and if in addition an increased quantity of the poison be inhaled, more or less acute symptoms follow. The localization of the deposit of lead is therefore of some importance.
Meillère and Richer[4] give an analysis of various organs of the body, but their results are not in accord with the majority of other observers. They found that the hair particularly contains a large quantity of lead. They do not seem to have examined the bones. Next to the hair, the liver seems to have contained the largest amount. Wynter Blyth[5] found 117·1 milligrammes of lead in the brain of a person who died of encephalopathy. In another case he found 0·6 gramme in the liver, 0·003 in the kidney, and 0·072 in the brain. Hougounencq[6] examined the organs of a person who died from lead poisoning, and found the largest amount of lead in the large intestine.
| Large intestine | 0·2150 | gramme. |
| Small intestine | 0·0430 | „ |
| Liver | 0·0050 | „ |
| Brain | 0·0008 | „ |
In the lung, stomach, kidney, and heart, only traces were found.
Dixon Mann[7] describes some experiments in which potassium iodide was given in cases of chronic poisoning, and during the whole of the experiments the fæces and urine were analyzed three times a week. He found by this means that a considerable amount of lead was being eliminated by the intestine. He therefore administered 2 grammes of lead acetate three times a day for five days to a patient, and he found that the fæces contained 0·1762 gramme the first day, 0·17411 gramme the second day; the fourth day it had fallen to 0·0053 gramme, and on the sixth day to 0·0006 gramme. The largest amount at any one time in a day obtained from the urine was only just over 0·001 gramme; the average amount found in the case of chronic poisoning was[33] about 3 milligrammes, whereas the greatest amount at any one time in the urine was only 0·9 milligramme.
The quantity of lead present in the brain necessary to determine acute poisoning is not known, and it is probable that an extremely minute quantity will produce very serious effects; and in support of this may be quoted a number of observations in which search has been made for the metal in persons who have died of diseases affecting the brain associated with other symptoms of poisoning, and yet post-mortem examination of the brain by chemical methods has not revealed the presence of any lead whatever. In the case reported by Mott (see p. 71), no lead at all was recognized in the brain.
There are no reasons, therefore, for supposing that the immunity to lead poisoning depends on the fixation and storing up of the poisonous metal in a non-poisonous form in some special situation in the body, and, further, the particular situation in the body richest in lead in any given case of poisoning will depend rather on (1) the type of compound causing the poisoning, and (2) the portal through which such poisoning occurs.
The question of the detection of lead in the body is referred to in the chapter dealing with Chemical Examination. It is as well to point out in this connection that chemical investigation of the amount of lead present in the organs of persons dying from lead poisoning should, if possible, always be made where there is any doubt as to the diagnosis.
Certain observers—amongst them Gautier[8]—are of opinion that traces of lead may be found in normal persons. Thus, in a rat (Mus decumanus) Gautier found 2 milligrammes of lead in 60 grammes of liver. He considers that in many persons at least 0·5 milligramme of lead may be swallowed daily incorporated with the food, as a number of foods are liable to contamination by lead. Tinned foods, particularly those which are soldered up after the materials have been placed in the tin, certain tinned fruits with acid juices, often contain small masses of solder loose in the tins; in the case particularly of fruits the natural acid may slowly dissolve the lead from the solder. The amount of so-called “normal” lead, if it is to be found at all, must be very small, and would certainly be much smaller in the case of a normal person than in one who had been subject to definite lead poisoning. Such experimental evidence as is forthcoming supports[34] the clinical observations that persons exposed to small doses of lead eventually develop tolerance of the metal, so that they may ultimately withstand many times the dose sufficient in the first instance to produce poisoning.
Such circumstances are the natural factors in the prevention of poisoning, and if due care be given to their significance, the surgeon in charge of any lead works may by judicious treatment and alternation of employment so assist and strengthen the natural defensive forces that susceptibility may be diminished, and the degree of tolerance increased to a very considerable extent. We do not imply that efficiency in the exhaust ventilation can be in any way relaxed; all we desire to emphasize is that certain natural defensive forces of the body do undoubtedly exist by which susceptible persons ultimately become less susceptible, and that by appropriate means these defensive forces may be augmented.
Susceptibility and immunity to poisoning by lead may be considered, according to the type of lead compound absorbed, further in its relation to age and sex. All compounds of lead are not poisonous in the same degree; the more easily soluble compounds are more poisonous than the less soluble. On the other hand, compounds which appear at first sight unlikely to produce poisoning may do so; for instance, fritted lead or lead silicate, a substance largely used in the potteries as a glaze, and manufactured by fusing together litharge and a silicate, would appear at first sight to be quite an innocuous substance. Owing to its method of preparation, however, it is not a pure compound of lead and silica, but contains lead oxide, metallic lead, etc., entangled in its meshes, and experimentally one of us (K. W. G.) has demonstrated that such a compound may be acted on by the tissues of the body, both when injected subcutaneously and even when inhaled, and so gradually produce definite symptoms of lead poisoning, but at a much slower rate than the more poisonous lead compounds. The fineness of division in which the compound of lead exists is another factor affecting its poisonous nature; the more finely divided particles find their way into the lung more easily than the coarser particles. Various subsidiary matters may also determine the susceptibility in a given individual, and of these a certain number require mention, as they probably act as definite predisposing factors. Age and sex may be regarded as predisposing factors to lead poisoning, and certain diseases also.
[35]
—Young persons are regarded as more liable to lead poisoning than adults, although it is difficult to obtain definite figures on the point, the duration of employment acting as a disturbing factor in estimating the susceptibility of young persons. They may have worked in a lead works for a year or more without showing any signs of poisoning, but develop them later in adult life, although it is very likely that absorption had taken place during the earlier period. In the Report of the Departmental Committee on the Use of Lead in the Potteries (Appendix XII.), the attack rate for the period 1899 to 1909 for young persons is 19·3 per 1,000, and for adults 18·8 for the same period, but the figures upon which these attack rates are based are too small to build any conclusion. The general clinical conclusions of appointed surgeons and certifying surgeons in the various lead factories would be, we believe, that the susceptibility of young persons is at least twice that of adults, and there is some ground for supposing that the tissues of an adult when growth has ceased more readily adapt themselves to deal with the absorption and elimination of poisonous doses of lead than do the tissues of a young person.
—Women are more susceptible to poisoning by lead than men, and in lead poisoning from drinking water the proportion of women (especially pregnant women) and children attacked is stated to be higher than in men, and one such epidemic is quoted by Oliver where the rise in the number of miscarriages and premature births led to the discovery of the fact that the water-supply was contaminated with lead. The close relationship of lead poisoning to miscarriage has been repeatedly made out, especially by Oliver, in the white lead industry as carried on twenty years ago. Oliver also quotes the effect upon rabbits[9], Glibert upon guinea-pigs[10], and in the experiments of one of us (K. W. G.), referred to on p. 99, all the animals to which lead was given during pregnancy aborted; and, further, with one exception out of eight animals, all died of lead poisoning, not as the result of the abortion, but some time later, although no further administration of lead was made. This confirms the well-known abortifacient effect of diachylon, and there is no doubt that the lead circulating in the maternal blood determines the abortion. Further, observers who have examined the fœtus in such cases have demonstrated the presence of lead in the fœtus itself. Oliver[11] found that eggs painted with lead nitrate did not[36] hatch out, and on opening the eggs the embryos were found to have reached only a limited stage of development, and to have then died, whereas control eggs painted with lime produced live chicks. From what is stated later with regard to the curious action of lead upon the blood, the mechanism of abortion is easily understood; it is probable that placental hæmorrhages are produced, as in other organs of the body. But the effect of lead on the female is not only apparent during pregnancy. A considerable number of women working in lead processes suffer from amenorrhœa, and often from periods of menorrhagia and dysmenorrhœa, which as a rule is the more striking symptom. The effect of lead on the uterine functions, however, only exists so long as the constant intake of the poison is taking place, and many cases are recorded where women, after having had successive abortions while working in lead factories, have ultimately gone through a normal pregnancy and given birth to a living child. This circumstance bears a strong analogy to the similar train of events in syphilis.
In the Report of the Committee on the Use of Lead in the Potteries, some inquiry was made with regard to the possible association of lead absorption on the male side as a predisposing cause of infant mortality and premature birth. The tables given are not very conclusive, and from our own observations there seems to be very little evidence for supposing that a male lead worker is less likely to beget children, or that his children are more likely to be unhealthy than those of men working in any other industrial process. We are here speaking of the effect of lead under the conditions of its general use in this country now. In the absence of any precautions whatever as to daily absorption of dangerous dust, the effect on the offspring, even in the case of male lead workers, may well be evident, as has been shown by Chyzer[12] in the manufacture of pottery as a home industry in Hungary. One greatly disturbing factor in estimating the greater susceptibility of the female than the male in many lead industries is that the more dangerous work is performed by the women, such, for instance, in the Potteries, as the process of colour-blowing and ware-cleaning.
—In lead poisoning, as in many other diseases, a number of predisposing and contributory causes may be cited which tend to lower the susceptibility of the individual to the poisonous effect of the metal and[37] its compounds, or to so modify the functions of the body that a smaller dose of poison may produce more profound changes than would otherwise be the case.
Certain diseases may be regarded as predisposing causes by lowering the general resistance of the body tissues to the influence of lead, and a consideration of the chapter on Pathology will at once demonstrate how seriously certain diseases may contribute in this way.
The peculiar effect of lead is upon the blood and the walls of the bloodvessels, and it will therefore follow that any disease which may affect the intima of the bloodvessels may predispose to lead poisoning; and, further, as the elimination of lead takes place to a certain extent through the kidney, any disease which affects either the renal epithelium or the general maintenance of the excretory function of the kidney may predispose that organ to the irritative effects of the lead circulating in the blood. In the same way, the condition of lead absorption in which the balance of absorption and elimination of lead remains in such a ratio that no definite symptoms of lead poisoning appear may have that delicate balance easily upset by the introduction of some secondary cause, which, when operating in association with lead absorption, may precipitate symptoms attributable to poisoning by that metal. Chronic alcoholism especially, producing as it does definite changes in the kidney of itself—changes which it is impossible to distinguish by the naked eye from the effects of lead poisoning—must clearly act as a predisposing, if not even an exciting, cause of lead kidney infection. In experiments upon animals, it was found that the addition of alcohol to the diet of an animal which was the subject of chronic lead absorption precipitated the attack of definite poisoning; in other words, the latent period of lead poisoning—that is to say, the resistance exhibited by the tissues to the toxic influence of lead—was considerably diminished by this addition of a second irritant, alcohol. In several experiments, also, where the form of lead experimented with was one of the least toxic of the lead compounds, the animals subjected to such a compound alone did not become poisoned, but succumbed if alcohol were added to their diet. This experimental work is amply borne out by the clinical evidence of all persons who have had experience of industrial lead poisoning, as cases of colic and wrist-drop are frequently observed in lead workers shortly after alcoholic[38] excesses. Individuals, therefore, who are suspected of the alcoholic habit should not be employed in any process where they are likely to run risk of absorption of lead dust.
Such diseases as syphilis and gout, by causing a heightened arterial tension or definite disease of the intima of the bloodvessels themselves, tend to weaken the arteries in much the same manner as does lead circulating in the blood, and must on that account act as predisposing causes.
In persons employed in lead trades some species of tolerance is generally developed, and if the functions of the body progress in the normal way the balance of elimination and absorption are equal, and, as will be seen later, the chief channel for the elimination of lead from the body is through the bowel. It follows, therefore, that any disease which tends to produce constipation or chronic inactivity of the normal intestinal functions will also tend to lower the resistance of the individual to lead poisoning.
Of the various types of intestinal disease of a chronic nature—such, for instance, as chronic dysentery, colitis, and the like—little need be said; but the predisposing effect of diseased conditions of the upper portion of the alimentary canal must not be overlooked, more particularly affections of the oral cavity itself. This special type of infection, often included under the term of “oral sepsis,” besides producing anæmia, is also a constant cause of intestinal disturbance, and as such operates as a particular predisposing cause of lead poisoning.
With regard to gout the evidence is not so clear. It was pointed out by Garrod[13] that gout was common among house-painters, and it has been generally stated that lead poisoning predisposes to this complaint. In the opinion of a considerable number of observers, however, gout is by no means common among persons working in white lead factories or lead-smelting works, but there seems to be some reason to suppose that it is somewhat common among those persons employed in the painting trades, but not among those employed in the manufacture of paints and colours. From the experiments carried out by one of us [K. W. G.[13]]. it seems probable that the occurrence of gout among painters may be associated with the use of turpentine, largely employed in the ordinary processes of painting, as this substance in particular is not one that is used by workers in[39] other lead trades, and, from experiments performed on animals, the inhalation of turpentine vapour was found to produce very definite changes both in the kidney and the general metabolism of the body.
—Malnutrition is recognized as a predisposing cause of practically all forms of disease, and with a chronic intoxication, such as lead poisoning, malnutrition and starvation, with its attendant depression of all the vital forces of the body, is essentially a predisposing cause of poisoning, so much so that even the fact of commencing work without previously partaking of food may operate directly as a cause of poisoning. It has been found, moreover, experimentally by one of us [K. W. G.[14]] that an animal fed with milk containing lead nitrate did not develop poisoning, though the control animal developed well-marked symptoms of poisoning with a much smaller dose given in water.
—Anæmia has already been referred to as occurring with great frequency in persons who are absorbing lead, and it usually forms one of the chief factors in the symptom-complex of lead cachexia. As the action of lead is particularly upon the blood and the hæmopoietic organs, diminishing the number of red cells and the amount of hæmoglobin, and impairing the organs from which fresh blood-cells are produced, a disease or state associated with anæmia other than of lead origin acts as a definite predisposing cause in the development of toxic symptoms in a worker in an industrial lead process.
Among the anæmias, two particular types may be referred to as of chief importance. In the first place, chlorosis, the anæmia occurring particularly in young women, is often associated with intestinal stasis. Lead anæmia occurring in a chlorotic person is always more severe than simple lead anæmia. Young persons suffering from chlorosis, therefore, should not be employed in a dangerous lead process until the anæmia has been treated. The second type of anæmia, which, from its frequency, may be also regarded as a predisposing cause of lead poisoning, is chronic secondary septic anæmia. Anæmias of this type, as was pointed out by William Hunter[15], resemble in many points the original idiopathic or Addisonian anæmia, often termed “pernicious anæmia,” and one of us has had occasion to inquire into the curious type of secondary anæmia associated with septic affections of the upper respiratory tract, particularly those related[40] to chronic suppurative affections of the accessory sinuses of the nose, of the gums, of the mucous membrane of the mouth and the throat. The commonest forms of this secondary anæmia are those due to chronic post-nasal discharge, and to chronic infections of the gums and alveolus of the jaws, the latter often classed together under the term “pyorrhœa alveolaris.” This term is an exceedingly clumsy one, indicating a discharge of pus from the gum edges and sockets of the teeth, which are often loose. The disease commences as an infective gingivitis along the edges of the gum, and progresses to rarefying osteitis of the alveolar process, and often of the body of the bone. The affection rarely gives rise to pain, and as a rule the individual is entirely unaware that any chronic suppuration is present, and little or no notice is therefore taken of the disease. Progressive anæmia may thus be set up without any knowledge of its cause, partly by absorption of the actual bacteria and their products through the alveolar bloodvessels, and partly by the fact of the constant swallowing of pus and bacterial products, which set up various forms of chronic gastro-intestinal incompetence. From the discharges of the mouth, and issuing from the gum edges, numerous bacteria have been isolated, and in more recent work one of us [K. W. G.[16]] has succeeded in isolating and identifying certain bacteria as a direct cause of arthritis deformans, a malady occasionally, but without sufficient grounds, ascribed to lead poisoning. Arthritis of various types may occur in persons engaged in lead trades, but in all such cases we have had the opportunity of examining there has been some obvious source of septic infection, and no evidence that the arthritis was due to the action of lead. It is most important to draw the attention of those engaged in the protection of lead workers from the dangers of their occupation to these chronic septic conditions of the mouth, and it may be taken as a general rule that, wherever the blue line makes its appearance along the gums, such gums are in a state of chronic infection, and the appearance of the blue line is merely a secondary effect. It is exceedingly rare to find the blue line in persons with intact gums and clean teeth; and although attention is frequently drawn to the fact that a lead line exists in a person whose teeth are normal, little or no notice is taken of the presence or absence of a suppurative condition of the gum margins. Moreover, such a suppurative condition does not always result in obvious inflammation of the gum edges,[41] and very considerable destruction of the alveolus and the interdental bone may exist without any obvious signs of its presence, unless the case be examined carefully with a fine probe. This particular point has been the subject of experiment by one of us. Animals exposed to the influence of air laden with lead dust never develop a blue line, although all the usual symptoms of lead poisoning make their appearance. When, however, some slight suppurative lesion of the gums was produced by an inoculation into the gum tissue of organisms isolated from a case of infective gingivitis in a human being, the site of inoculation and any suppurative lesion that resulted locally at once allowed the development of a blue line, and it was only in animals so treated that it was possible to produce experimentally the Burtonian line.
There is no doubt that any chronic septic infection may predispose to lead poisoning through the production of a secondary anæmia, and it is therefore inadvisable to pass for work in a lead process of a dangerous nature any persons suffering from an infected condition of the mouth. It follows also that the care of the mouth and gums should be rigorously enforced upon all persons employed in lead trades, as the mere mechanical facilities for the accumulation of débris around the individual teeth tends to increase the quantity of lead dust that may be retained in the mouth. This is gradually rendered soluble and absorbed, through the action of the bacterial acids which are always produced along the gum margins when any entangled food is retained in the interdental spaces.
One further point of importance attaches to the infections of the upper respiratory tract—namely, the constant ingestion of bacteria of a fermentative type. By this means the contents of the stomach may be maintained in a state of hyperacidity, and any small quantities of lead which become swallowed are thereby at once rendered soluble in the intermeal periods.
Of the other types of anæmia which may act as predisposing causes of lead poisoning, little need be said, as they are either associated with other grave symptoms or are rare in this country. But as all forms of anæmia, particularly septic anæmia, malarial fever, etc., are associated with destruction of the blood-cells, the presence of basophile staining granules in the red corpuscles of such persons is a constant feature, and must not be confounded[42] with the basophile staining owing its origin to the effect of lead.
In addition to the diseases mentioned which may be said to predispose to lead poisoning, certain other diseases have been stated to be predisposed to by the action of lead. It is no doubt a fact that where chronic anæmia, wasting, loss of subcutaneous fat, decreased muscular power, and general lowering of the metabolic activity of the body, are produced, an individual so affected may be supposed to be more susceptible to certain infectious diseases, and among these stress has been laid on the alleged association of phthisis with lead absorption. This point is discussed in the next chapter.
In summing up the difficult question of predisposition to lead poisoning, together with the correlated questions of susceptibility and immunity, certain facts may at any rate be clearly stated:
1. Undoubted individual susceptibility and immunity exist with regard to lead poisoning in exactly the same way as individual susceptibility and immunity may be shown to exist towards poisoning by many other metals and drugs. Therefore, given the same opportunities for infection, a person showing early signs of lead absorption may be regarded as susceptible.
2. Females are at least twice, and probably three times, as susceptible to lead poisoning as are males. Much of this susceptibility is determined by the extra stress thrown upon the female generative organs.
3. Certain diseases predispose to lead poisoning mainly by nature of the alterations in metabolism produced—chiefly anæmia.
4. Many persons engaged in lead industries become gradually tolerant of the absorption of lead, and in time resist much larger doses than would have been possible at the commencement of exposure, but in such persons the balance between absorption and excretion upon which that tolerance depends may become easily disturbed by intercurrent disease or sudden increase in absorption.
[43]
[1] Cloetta: Dixon Mann’s Forensic Medicine and Toxicology, p. 463.
[2] Oliver, Sir T.: Diseases of Occupation, p. 142.
[3] Goadby, K. W.: Departmental Committee on Lead Poisoning, etc., in China and Earthenware Manufacture, Appendix No. XXV.
[4] Meillère and Richer: Meillère’s Le Saturnisme. Paris, 1903.
[5] Blyth: Abstract of Proc. Chem. Soc., 1887-88.
[6] Hougounencq: Meillère’s Le Saturnisme, p. 73.
[7] Dixon Mann: British Medical Journal, 1893.
[8] Gautier: Société de Biologie, April, 1903.
[9] Oliver, Sir T.: British Medical Journal, May 13, 1911, p. 1096.
[10] Glibert, D. J.: Le Saturnisme Expérimental. Extrait des Rapports Annuels de l’Inspection du Travail. Bruxelles, 1906.
[11] Oliver, Sir T.: Diseases of Occupation, p. 139.
[12] Chyzer, A.: Des Intoxications par le Plomb se présentant dans la Céramique en Hongrie. Budapest, 1908.
[13] Garrod: The Lancet, 1870.
[14] Goadby, K. W.: Departmental Committee on Lead Poisoning, etc., in China and Earthenware Manufacture, Appendix XXV.
[15] Hunter, William: Severest Anæmias.
[16] Goadby, K. W.: The Lancet, March 11, 1911.
[44]
[A] Based mainly on reports received from certifying factory surgeons during the ten years 1900-1909.
Classification of notified cases of lead poisoning was carried out on practically the same lines between the years 1900 and 1909, and comparison of the data so collected has interest, in view of their large number—nearly 7,000—in respect of (1) increase or decrease in recorded amount in each one of eighteen classes of industries; (2) severity and number of attack—i.e., whether first, second, third, or chronic; and (3) main symptoms.
Notification was first enjoined by Section 29 of the Factory and Workshop Act, 1895, which subsequently, on consolidation of the Factory Acts, became Section 73 of the Act of 1901. This enactment requires every medical practitioner, attending on, or called in to visit, a patient whom he believes to be suffering from lead poisoning contracted in a factory or workshop, to notify the case forthwith to the Chief Inspector of Factories at the Home Office; and a similar obligation is imposed on the occupier of a factory or workshop to send written notice of every such case to the certifying surgeon and inspector of factories for the district. In form there is close similarity between this section and that requiring notification under the Infectious Diseases (Notification) Act; but whereas the symptoms of these diseases are, within well-recognized limits, precise, in lead poisoning the differential diagnosis has not infrequently to be made from a variety of common ailments—headache, anæmia, rheumatism, abdominal pain; and there is no precise standard of what constitutes lead poisoning.
The notification of the practitioner as a rule gives no information beyond the belief that the case is one of lead poisoning. As a matter of routine the notification is followed up by an inquiry[45] by the certifying surgeon and inspector to see whether regulations already in force have been infringed in the particular work-place or not, and as to how far there may have been contributory negligence on the part of the sufferer. The data supplied on the surgeon’s report form the basis of the tabulation[1]. Brief explanation is wanted of the method adopted in classification. Cases represent all attacks reported within a year, and not previously reported within the preceding twelve months, so as to make the number of persons and cases in a year the same. Where the interval between two reports on the same person was more than twelve months, the fresh attack was again included. The number of such second reports on persons already included in a return numbered 284 (4·2 per cent.), and a portion of these certainly, probably not more than 100, have been included twice or thrice in the total 6,638 cases. Cases in which there was obvious error in diagnosis, or in which the opinion of the certifying surgeon was very strongly against the diagnosis (especially when the report had been made in the first instance by the occupier alone, and not by a medical practitioner), were excluded from the return. These numbered 458 (6·8 per cent.). Others, again, where there was a strong element of doubt, but not to be regarded as more than a difference of opinion between two medical men, were marked doubtful and included. Of these there were 424 (6·3 per cent.).
The classification of industries was designed to represent the way in which the poisoning may be supposed to originate from (a) lead fumes (1 to 4), (b) handling metallic lead (5 and 6), (c) dust from lead compounds (7 to 14), and (d) lead paint (15 to 17). We attach now only slight importance to this attempt to define causation, as it will appear from our survey that we regard almost all cases as the result of inhalation either of fumes or dust.
The reports describe not only the particular attack, but also the general condition of the patient at the time of the attack. Very frequently a combination of symptoms—colic, anæmia, and varying degree of paralysis—are described as present, and when this is the case each one of them has been entered under the appropriate heading. The total number of symptoms, therefore, greatly exceeds the number of cases, but this does not affect the correctness of the estimate of each one as a proportion on the total number reported. The reports do not give detailed[46] information such as can be gained from hospital records. Especially is this the case with the symptoms of paralysis and encephalopathy.
Table III. shows the number of reported cases included in returns for each of the years 1900 to 1909. On the total figures there has been a reduction of 47·7 per cent. In the several industries the salient feature is that the considerable diminution achieved is limited to industries—notably white lead, earthenware and china, litho-transfers, and paints and colours—in which, under regulations or special rules, locally applied exhaust ventilation for the removal of dust, and periodical medical examination of the workers, have been required. Where, owing to the nature of the processes carried on, it has been found impracticable, in the present state of knowledge, to apply local exhaust ventilation, and where periodical examination of the workers is lacking, as in smelting of metals[A] and industries using paint, there has been tendency to increase in the number of cases. In coach-building the increase is in part due to activity in the motor-car industry.
[A] This is now required by the regulations dated August 12, 1911.
[47]
TABLE III.—NOTIFICATION OF POISONING BY LEAD (under S. 73, 1901), 1900-1909.
| Industry. | Reported Cases. | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total 1900-09. |
1909. | 1908. | 1907. | 1906. | 1905. | 1904. | 1903. | 1902. | 1901. | 1900. | ||||||||||||||
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) | |||||||||||||
| Lead poisoning | 6,762 | 275 | 553 | 30 | 646 | 32 | 578 | 26 | 632 | 33 | 592 | 23 | 597 | 26 | 614 | 19 | 629 | 14 | 863 | 34 | 1,058 | 38 | ||
| 1 | . | Smelting of metals | 412 | 18 | 66 | 5 | 70 | 2 | 28 | 2 | 38 | 1 | 24 | 1 | 33 | 1 | 37 | 2 | 28 | 54 | 3 | 34 | 1 | |
| 2 | . | Brass works | 75 | 4 | 5 | 6 | 9 | 1 | 11 | 5 | 1 | 10 | 1 | 15 | 5 | 6 | 1 | 3 | ||||||
| 3 | . | Sheet lead and lead piping | 109 | 3 | 9 | 2 | 14 | 6 | 7 | 9 | 7 | 11 | 12 | 17 | 17 | 1 | ||||||||
| 4 | . | Plumbing and soldering | 217 | 12 | 28 | 27 | 20 | 2 | 16 | 4 | 24 | 2 | 21 | 3 | 26 | 23 | 1 | 23 | 9 | |||||
| 5 | . | Printing | 200 | 17 | 21 | 1 | 30 | 2 | 26 | 3 | 16 | 2 | 19 | 4 | 15 | 13 | 2 | 19 | 23 | 1 | 18 | 2 | ||
| 6 | . | File-cutting | 211 | 19 | 8 | 9 | 2 | 10 | 15 | 12 | 20 | 4 | 24 | 2 | 27 | 1 | 46 | 7 | 40 | 3 | ||||
| 7 | . | Tinning and enamelling | 138 | 2 | 21 | 10 | 25 | 18 | 1 | 14 | 1 | 10 | 14 | 11 | 10 | 5 | ||||||||
| 8 | . | White lead | 1,295 | 31 | 32 | 2 | 79 | 3 | 71 | 108 | 7 | 90 | 1 | 116 | 2 | 109 | 2 | 143 | 1 | 189 | 7 | 358 | 6 | |
| 9 | . | Red lead | 108 | 10 | 12 | 7 | 6 | 10 | 11 | 6 | 13 | 14 | 19 | |||||||||||
| 10 | . | China and earthenware | 1,065 | 57 | 58 | 5 | 117 | 12 | 103 | 8 | 107 | 4 | 84 | 3 | 106 | 4 | 97 | 3 | 87 | 4 | 106 | 5 | 200 | 8 |
| 10 | a. | Litho-transfers | 48 | 1 | 2 | 10 | 5 | 5 | 3 | 3 | 2 | 7 | 10 | |||||||||||
| 11 | . | Glass cutting and polishing | 48 | 9 | 4 | 2 | 3 | 1 | 4 | 4 | 1 | 3 | — | 4 | 8 | 2 | 11 | 3 | 7 | |||||
| 12 | . | Enamelling iron plates | 52 | 1 | 3 | 7 | 6 | 4 | 2 | 3 | 4 | 3 | 1 | 9 | 11 | |||||||||
| 13 | . | Electric accumulators | 285 | 6 | 27 | 2 | 25 | 1 | 21 | 26 | 27 | 1 | 33 | 28 | 16 | 1 | 49 | 1 | 33 | |||||
| 14 | . | Paints and colours | 422 | 7 | 39 | 2 | 25 | 35 | 1 | 37 | 57 | 1 | 32 | 1 | 39 | 1 | 46 | 56 | 56 | 1 | ||||
| 15 | . | Coach-building | 697 | 41 | 95 | 6 | 70 | 3 | 70 | 3 | 85 | 7 | 56 | 3 | 49 | 4 | 74 | 5 | 63 | 1 | 65 | 4 | 70 | 5 |
| 16 | . | Ship-building | 269 | 10 | 27 | 1 | 15 | 22 | 1 | 26 | 1 | 32 | 2 | 48 | 24 | 1 | 15 | 1 | 28 | 1 | 32 | 2 | ||
| 17 | . | Paint used in other industries | 452 | 18 | 42 | 47 | 1 | 49 | 2 | 37 | 3 | 49 | 2 | 27 | 3 | 46 | 1 | 44 | 1 | 61 | 50 | 5 | ||
| 18 | . | Other industries | 659 | 20 | 57 | 2 | 78 | 5 | 56 | 2 | 66 | 2 | 70 | 1 | 53 | 3 | 40 | 64 | 89 | 1 | 86 | 4 | ||
The principal figures are those of the cases, fatal and non-fatal; the small figures relate to fatal cases only.
For the sake of completeness the figures for the years 1910 and 1911 are given below. The grand totals are comparable with those for each of the years 1900 to 1909, but not the total for all of the several groups of industries. Thus, the name of heading No. 7 is altered to “Tinning of metals,” and No. 12 to “Vitreous enamelling,” because of regulations widening their scope, and now including cases which previously figured in No. 18, “Other industries.”
| Industry. | 1911. | 1910. | ||
|---|---|---|---|---|
| Lead poisoning | 669 | 37 | 505 | 38 |
| Smelting of metals | 48 | 3 | 34 | 5 |
| Brass works | 9 | 1 | 7 | |
| Sheet lead and lead piping | 12 | 4 | ||
| Plumbing and soldering | 37 | 2 | 25 | 1 |
| Printing | 32 | 2 | 33 | 4 |
| File-cutting | 18 | 2 | 9 | 1 |
| Tinning of metals | 13 | 17 | ||
| Vitreous enamelling | 19 | 1 | 17 | |
| White lead | 41 | 2 | 34 | 1 |
| Red lead | 13 | 1 | 10 | |
| China and earthenware | 92 | 6 | 77 | 11 |
| Litho-transfers | 1 | 1 | ||
| Glass cutting and polishing | 5 | — | ||
| Electric accumulators | 24 | 1 | 31 | |
| Paints and colours | 21 | 17 | 1 | |
| Coach and car painting | 104 | 5 | 70 | 6 |
| Ship-building | 36 | 6 | 21 | 2 |
| Use of paint in other industries | 56 | 1 | 51 | 3 |
| Other industries | 88 | 4 | 47 | 3 |
TABLE IV.—ANALYSIS OF REPORTS ON LEAD POISONING BY CERTIFYING SURGEONS FROM JANUARY 1, 1900, TO DECEMBER 31, 1909.
To reduce the size of the table, columns showing the number in each occupation in which (a) the severity of attack, and (b) the number of attack were not stated, have been omitted. Of the former there were 170, and of the latter 245. The total figures, however, in Column 3 include them.
[50]
Table IV. shows the severity of the attacks as stated by the surgeon, the number of attack, and the main symptoms. The personal element enters into the character of the reports, and symptoms which one surgeon might describe as slight another might regard as moderate, or even severe. In general, however, “slight” includes cases of (1) colic without complication, and of comparatively short duration; (2) anæmia in adolescence aggravated by employment; and (3) either of the above with tendency to weakness of the extensors. “Moderate” includes (1) a combination of colic with anæmia; (2) profound anæmia; (3) partial paralysis; and (4) cases in which there is constitutional debility. “Severe” includes (1) marked paralysis; (2) encephalopathic conditions—convulsions, optic neuritis, and mental affections; (3) grave undermining of the constitution associated with paralysis, renal disease, and arterio-sclerosis. The reports are made during the attack, and information is not received of the sequelæ which may supervene, except in the event of a later report as the result of fresh exposure to lead. Number of attack has reference to definite occurrence of disability. Transient attacks which have preceded the disabling condition have been usually disregarded. It was necessary to limit the number of attacks which might be regarded as indicating chronic plumbism, and all those included in Column 10 are either third attacks or cases of chronic lead poisoning. Among the main symptoms, the headings “Gastric,” “Paretic,” “Encephalopathic,” and “Rheumatic or Arthralgic,” represent fairly accurately the relative incidence of these in cases of lead poisoning in this country; those under the headings “Anæmia” and “Headache” are useful in comparing relative incidence on the two sexes, but they occur, probably, much more frequently than the figures would indicate; those under “Tremor” and “Other” are less valuable. Under “Other” are included “Gout,” “Nephritis,” or “Cerebral Hæmorrhage,” so that entry under this head indicates chronic, rather than mild, lead poisoning. The conclusions from the table are easy to draw, as, in general, the feature which causes severity of symptoms to be prominent leaves its mark also on “Number of Attack” and “Main Symptoms.” Thus, in the industries in which severe cases exceed the average (brass, plumbing, printing, file-cutting, tinning, glass-cutting, ship-building, paints used in other industries, and other industries), the chronic nature of the plumbism is markedly above the average, and some severe symptom, usually paralysis, is also above the average. An exception to this rule is china and earthenware, where severity is considerably below the average, but where, among men, the figures for chronic lead poisoning and paralysis are distinctly high. It will be seen, however, that the proportion of slight cases even in this industry is below the average. On the other hand, severity is below the average in smelting, white lead, red lead, litho-transfers, enamelling, electric accumulators, paints and colours, and coach-painting, and the symptoms in these industries are, in general, colic rather than high degree of paralysis; but in them a severe symptom which is above the average, in general, is encephalopathy. The explanation of these differences depends, we believe, on two factors: (1) Duration of employment, with which, naturally, the age of the worker is associated; (2) opportunity of inhaling lead dust. The longer the employment, the more likely, naturally, if absorption goes on, is the plumbism to become chronic, and to be associated with paralysis, its prominent sign. Duration of employment among males in file-cutting and china and earthenware, as contrasted, for instance, with that in white lead, is very much longer, and the same could be shown of comparatively[51] new industries, such as electric accumulators and litho-transfers. Thus, in one year the age distribution and duration of employment of those attacked in three of these industries was as follows:
| Industry. | Age Distribution. |
Duration of Employment. |
||
|---|---|---|---|---|
| Under 30. |
Over 30. |
Under 5 Years. |
Over 5 Years. |
|
| Per Cent. | Per Cent. | Per Cent. | Per Cent. | |
| China and earthenware | 59·4 | 40·6 | 52·2 | 47·8 |
| White lead | 45·7 | 54·3 | 86·8 | 13·2 |
| File-cutting | 22·9 | 77·1 | — | 100·0 |
Persons employed in the manufacture of white and red lead, electric accumulators, paints and colours, and the others named, are exposed essentially to dust from salts of lead, which are readily absorbed. Poisoning, therefore, if precautions are inadequate, will quickly show itself, causing certain workers to seek other employment after one attack. Poisoning thus produced is more likely to induce colic, or, if the dose has been large or the individual markedly susceptible, encephalopathic symptoms, than paralysis. On the other hand, the slowness of the onset of symptoms in the case of brass workers, plumbers, printers, file-cutters, and tinners, is more the result of inhalation of fumes or of dust of metallic lead than of salts of lead; or if the inhalation be of salts of lead, then of these in less amount and over a long period, with, as a result, gradual undermining of the constitution, showing itself in paralysis, arterio-sclerosis, and renal disease. The two factors indicated obviously account for the differences in severity and number of attack between males and females. If second and third attacks are comparatively fewer in females than in males, it follows that, in general, the attack will be less severe also, and this is brought out in the figures. Cerebral symptoms—encephalopathy, to which headache may be added—are more than twice as frequent in females as males. This may be due to idiosyncrasy, but it may very possibly be simply the result of short duration of employment of young workers in processes where dust of salts of lead is incidental.
Attacks generally are most frequent in the first or second year of employment. Thus, of 2,195 attacks reported in the four years 1904 to 1907, as to which sufficient data are given, 898[52] occurred in the first two years of employment, and of these 672 occurred in the first year—that is, three-sevenths of all the cases were reported during the first two years, and four-sevenths in the whole of the remaining years of employment. It is, unfortunately, impossible to say what is the proportion of attacks among those employed for any given age period. In some factories—as, for example, lead smelting works—the average duration of employment is about thirteen years. The length of employment preceding an attack was made out from reports on cases which occurred in the white lead industry in 1898—a time when a number of new workers were taken on to replace the female labour abolished in June of that year, and conditions as regards removal of dust were entirely different from what they are now. The figures, therefore, can only be considered to have bearing upon incidence under almost the worst possible circumstances. Of 155 attacks, duration of employment was stated to have been less than 1 week in 3, from 1 week to 1 month in 8, from 1 to 3 months in 62, from 3 to 6 months in 44, from 6 to 12 months in 12, and 1 year and over in 26.
Attempt has been made to discredit the value of Section 73 of the Factory Act, 1901, on the ground that the proportion of cases in which some degree of paralysis is present is very high as compared with the extent found by other observers. The points we have laid stress on—(1) duration of employment, (2) varying kinds and amounts of lead dust and fumes—are, we believe, quite sufficient to account for, and give value to, the figures dealt with. To them should be added another factor, though one of less account—namely, the extent to which particular muscles are used. In the case of file-cutters, for instance, there is no doubt that the cramped position of the left hand holding the chisel, and the work thrown on the right in holding the heavy mallet, determine the direction of the paralysis, especially on to the muscles of the thenar and hyperthenar eminences and of the fingers.
There is, however, difficulty in deciding whether such entries on reports as “weakness of arms and legs,” “weakness of arms,” “muscular weakness,” etc., should be interpreted as incipient paralysis.[A] With a disease like lead poisoning showing[53] marked tendency to affect the muscles supplied by the musculo-spiral and other nerves, the only safe course was to include all these terms as equivalent to partial paralysis. Table V. on p. 54 shows close parallelism for the six years.
[A] During the years 1910 and 1911 cases were classified so as to distinguish definite paralysis, as far as possible, from the more indefinite terms referred to, with the result tabulated opposite. We have little doubt that in most of the cases included in columns (3) and (6) some slight degree of paresis was present.
| Form of Paralysis. | 1910. | 1911. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Paralysis. | Weakness of Arms or Loss of Power. |
Total. | Paralysis. | Weakness of Arms or Loss of Power. |
Total. | ||||
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | |||
| Arms and legs | - | complete | — | — | — | 2 | — | 2 | |
| partial | 4 | 6 | 10 | 1 | 4 | 5 | |||
| Legs | - | complete | — | — | — | — | — | — | |
| partial | 4 | 4 | 8 | — | 6 | 6 | |||
| Both forearms | - | complete | 15 | — | 15 | 27 | — | 27 | |
| partial | 19 | 30 | 49 | 20 | 44 | 64 | |||
| Right forearm | - | complete | 8 | — | 8 | 5 | — | 5 | |
| partial | 6 | 4 | 10 | 4 | 7 | 11 | |||
| Left forearm | - | complete | 3 | — | 3 | 2 | — | 2 | |
| partial | 2 | 1 | 3 | 1 | 7 | 8 | |||
| Fingers | 3 | — | 3 | 7 | — | 7 | |||
| Neuritis (including numbness of hands or arms) | 5 | — | 5 | 5 | — | 5 | |||
| Other (including paralysis of deltoid, muscles of speech, locomotor ataxy, and general paralysis) | 1 | — | 1 | 4 | 2 | 6 | |||
| 70 | 45 | 115 | 78 | 70 | 148 | ||||
If it is difficult to distinguish rightly all the cases classed as “paralysis,” it is even more difficult to determine what should be included under the term “encephalopathy.” We have limited it to epileptiform seizures, optic neuritis (uncomplicated by epilepsy), and various forms of insanity. Table VI. on p. 54 is interesting as showing how fairly constant the numbers are from one year to another.
Except in the one industry of earthenware and china, in which a return of the number of persons employed according to process and kind of ware has been made on three separate occasions, and in which the reports of the certifying surgeons enable the cases of poisoning to be classified in the same way, it is difficult to determine accurately the attack rate of lead poisoning. Even in the earthenware and china trade many things have to be borne in mind. The poisoning which occurs is not distributed evenly over all the factories. Thus, among the 550 potteries, in the years 1904 to 1908, five potteries were responsible for 75 cases, and 173 for the total number of cases (517), leaving 377 factories from which no cases were reported.
[54]
Table V.—Forms of Paralysis: 1904-1909.
| Form of Paralysis. | Total. | 1909. | 1908. | 1907. | 1906. | 1905. | 1904. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | |||
| Arms and legs | - | complete | 12 | 2 | 2 | 1 | 2 | 1 | 4 | |
| partial | 62 | 13 | 7 | 9 | 13 | 9 | 11 | |||
| Legs | - | complete | 3 | — | — | 1 | — | 1 | 1 | |
| partial | 25 | 5 | 7 | 1 | 3 | 5 | 4 | |||
| Both forearms | - | complete | 162 | 29 | 33 | 29 | 28 | 24 | 19 | |
| partial | 334 | 59 | 70 | 56 | 56 | 43 | 50 | |||
| Right forearm | - | complete | 39 | 11 | 6 | 7 | 4 | 8 | 3 | |
| partial | 62 | 9 | 17 | 14 | 11 | 5 | 6 | |||
| Left forearm | - | complete | 14 | 2 | 2 | 4 | 1 | 3 | 2 | |
| partial | 22 | 4 | 1 | 4 | 6 | 4 | 3 | |||
| Fingers | 36 | 3 | 3 | 7 | 10 | 6 | 7 | |||
| Neuritis (including numbness of hands or arms) | 32 | 7 | 8 | 3 | 3 | 5 | 6 | |||
| Other (including paralysis of deltoid, muscles of speech, locomotor ataxy) | 10 | 3 | 1 | 3 | 1 | — | 2 | |||
| 798 | 147 | 157 | 139 | 138 | 114 | 118 | ||||
Table VI.—Encephalopathy.
| Symptom. | 1911. | 1910. | 1909. | 1908. | 1907. | 1906. | 1905. | 1904. |
|---|---|---|---|---|---|---|---|---|
| Epilepsy | 6 | 16 | 12 | 15 | 14 | 11 | 12 | 15 |
| Optic neuritis | 2 | 3 | 3 | 2 | 3 | 7 | 5 | 4 |
| Mental defect | 5 | 2 | 2 | 1 | 6 | 3 | 1 | 2 |
| Total | 13 | 21 | 17 | 18 | 23 | 21 | 18 | 21 |
The same state of things is found in all the other industries. Particular factories, owing to special method of manufacture or special manner of working, may have an incidence out of all proportion to that prevailing in the trade generally. And it is, of course, control of these more obvious sources of danger by the efforts of manufacturers and the factory inspectors that[55] has led to the notable reduction recorded—e.g., in white lead works and the pottery industry.
Returns of occupiers do not lend themselves readily to exact estimate of the number of persons exposed to risk of lead poisoning, as they do not differentiate the processes, and in nearly all factories in which lead is used some of those returned will not come into contact with it.
In industries, however, in which there is periodic medical examination of persons employed in lead processes an attack rate can be made out. It must be regarded as approximate only, as in the manufacture of electric accumulators, for instance, medical examination is limited to persons employed in pasting, casting, lead-burning, or any work involving contact with dry compounds of lead, whereas the reported attacks include a few persons engaged in processes other than those named.
Table VII.—Attack Rate from Lead Poisoning in the Year 1910 in Certain Industries.
| Industry. | Number of Exami- nations. |
Probable Number of Persons employed. |
Number of Reported Cases. |
Attack Rate per Thousand. |
|---|---|---|---|---|
| White lead | 77,752 | 1,495 | 34 | 22 |
| Red lead | 8,096 | 675 | 10 | 15 |
| Vitreous enamelling | 3,064 | 766 | 17 | 22 |
| Tinning of metals | 1,475 | 492 | 17 | 34 |
| Electric accumulators | 13,065 | 1,089 | 31 | 28 |
| Paints and colours | 19,081 | 1,590 | 17 | 11 |
| Earthenware and china | 78,560 | 6,547 | 77 | 12 |
As has been mentioned above, the accurate information we have of the numbers employed in the several processes in the earthenware and china industry enable us to use the figures for that industry to illustrate, what is certainly true of all other lead industries also, the fact of the relative greater degree of risk in one process than another.
The fall in the number of fatal cases attributed to lead poisoning, as is perhaps to be expected, seeing that the great majority are deaths from chronic lead poisoning, does not run parallel with the diminution in the number of cases. Thus, in the five years 1905 to 1909 the deaths numbered 144, as compared with 131 in the previous five years, although the cases fell from 3,761 to 3,001. We believe this is due to an increasing inclination to[56] attribute chronic nephritis, and even (without sufficient justification in our opinion) phthisis and pneumonia, to lead poisoning on the death certificates of lead workers. Copies of all death certificates on which lead poisoning is entered as directly or indirectly a cause are received by the Chief Inspector of Factories. All of industrial origin are included in the return. Of a total of 264 which could be followed up, encephalopathic symptoms appeared on the death certificate in 38 (10·6 per cent.); Bright’s disease, cerebral hæmorrhage, paralysis, or chronic lead poisoning either alone or as a combination of symptoms closely connected, in 188 (71·2 per cent.); phthisis in 13 (5·0 per cent.); and other diseases, such as pneumonia, etc., in 25 (9·4 per cent.). Table IX. brings out the relative frequency in the several groups of industries, and, as is to be anticipated, the different average age at death when due to acute and chronic lead poisoning.
TABLE VIII.—LEAD POISONING IN EARTHENWARE AND CHINA WORKS
(China, Earthenware, Tiles, Majolica, Jet and Rockingham, China Furniture and Electrical Fittings, Sanitary Ware).
| Processes. | Persons employed in 1907. |
Cases Reported: Average per Year. |
Attack-Rate per Thousand employed: Average per Year. |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1907- 1910. |
1903- 1906. |
1899- 1902. |
1907- 1910.[A] |
1903- 1906.[B] |
1899- 1902.[C] |
|||||
| In dipping-house: | ||||||||||
| Dippers | - | M. | 786 | 17 | 18 | 26 | 22 | 23 | 34 | |
| F. | 150 | 6 | 4 | 7 | 40 | 30 | 68 | |||
| Dippers’ assistants | - | M. | 463 | 3 | 3 | 7 | 7 | 7 | 15 | |
| F. | 397 | 13 | 18 | 17 | 33 | 46 | 45 | |||
| Ware-cleaners | - | M. | 115 | 1 | 2 | 3 | 9 | 20 | 30 | |
| F. | 461 | 15 | 18 | 30 | 33 | 41 | 65 | |||
| Total | - | M. | 1,346 | 21 | 23 | 36 | 15 | 17 | 27 | |
| F. | 1,008 | 34 | 40 | 54 | 34 | 42 | 58 | |||
| Glost-placers | - | M. | 2,291 | 16 | 12 | 33 | 7 | 5 | 14 | |
| F. | 120 | 1 | 1 | 1 | 8 | 10 | 14 | |||
| Majolica-painters | - | M. | 28 | — | — | — | — | — | — | |
| F. | 358 | 6 | 8 | 10 | 13 | 14 | 20 | |||
| Ground-layers | - | M. | 58 | 1 | — | 1 | 17 | — | 17 | |
| F. | 157 | 1 | 1 | 4 | 6 | 5 | 13 | |||
| Colour and litho dusters | - | M. | 14 | — | — | — | — | — | — | |
| F. | 143 | — | 1 | 4 | — | 7 | 33 | |||
| Enamel colour and glaze blowers | - | M. | 51 | — | — | 1 | — | — | 36 | |
| F. | 288 | 3 | 3 | 2 | 10 | 14 | 12 | |||
| Colour-makers and millers and mixers of glaze or colour | - | M. | 371 | 5 | 5 | 6 | 13 | 13 | 17 | |
| F. | 55 | 1 | 1 | 1 | 18 | 48 | 114 | |||
| Other persons in contact with lead | - | M. | 327 | 2 | 1 | 2 | 6 | 5 | 11 | |
| F. | 132 | 1 | 2 | 4 | 8 | 21 | 75 | |||
| Grand total | - | M. | 4,504 | 44 | 41 | 80 | 10 | 9 | 19 | |
| F. | 2,361 | 45 | 57 | 80 | 19 | 25 | 37 | |||
| (M. and F.) | 6,865 | 89 | 98 | 160 | 13 | 15 | 25 | |||
[A] Calculated on return of employment for 1907.
[B] Calculated on return of employment for 1904.
[C] Calculated on return of employment for 1900.
[57]
The statistical evidence from death certificates published in the decennial supplements of the Superintendent of Statistics[2] is of significance, not only in enabling comparison to be made between one industry and another, in regard to mortality from lead poisoning, but also in determining the other causes of death most frequently entered on death certificates of lead workers, and therefore, if they are in high excess, as compared with male workers generally, they are to be ascribed with some degree of certainty to deleterious effects of lead on some of the principal organs. Thus, in Table X. a list of occupations is given in which the mortality from plumbism in the years 1900 to 1902 was double or more than double the standard. It represents the mortality which would occur if the male population in the particular industry had exactly the same age population as that of “all males.” Further, the annual mortality among “all males” is taken as 1,000, and that of males engaged in the several industries is stated as a proportion of this. This “mortality figure” of 1,000 is made up of the mortality from various causes (of which only those considered to bear upon lead poisoning are given in the table) in the proportion stated.
The contention that, because lead workers die from certain diseases more frequently than “all males,” such diseases must be the sequelæ of lead poisoning is untenable unless other recognized causes of the diseases in question have been excluded. For excess of deaths from phthisis and respiratory diseases the conditions of work and exposure to inhalation of mineral and metallic dust or vitiation of atmosphere, in pottery, spelter, printing works, and file-cutting workshops, sufficiently account. The figures, indeed, take no account of this, and their value, in some at any rate, is still further diminished by the very large number of occupations (several involving no contact at all with lead) included in the headings. With exception of the strikingly greater proportion of deaths among lead-workers from Bright’s disease, the figures are too contradictory to draw deductions from as to what are “sequelæ” of lead poisoning. But this figure—160, as compared with 35 for all males—is confirmatory evidence, if any were needed, that chronic Bright’s disease is a sequela. And, from the pathology of lead poisoning, we believe that the granular condition of the kidney is due to the sclerotic change brought about in its substance by microscopic hæmorrhages. We have very little evidence indeed in man that this interstitial change is set up or preceded by an acute tubal nephritis. While we do not deny that there may be some parenchymatous change associated with lead poisoning, we do not believe that it is of the kind which gives rise to the large white kidney, and we should therefore exclude such disease as a sequela. But if chronic Bright’s disease is admitted, the train of symptoms associated with it—notably arterio-sclerotic changes resulting in cerebral hæmorrhage and albuminuric retinitis—must be admitted also. Unless it were established that granular nephritis were present in a lead-worker before commencement of lead employment, we think it would be useless to endeavour to prove that the condition was independent of lead, despite its comparative frequency as a cause of death apart from employment.
[58]
TABLE IX.—MAIN SYMPTOMS APPEARING AS THE CAUSE IN 264 DEATH CERTIFICATES OF LEAD POISONING.
[59]
TABLE X.—COMPARATIVE MORTALITY FROM SPECIFIED CAUSES AMONG MALES ENGAGED IN CERTAIN OCCUPATIONS: 1900-1902.
[60]
Other conditions which might readily be admitted as sequelæ are optic neuritis, following on an attack of encephalopathy. No general statement can be made in regard to mental and nervous diseases, gout, pernicious anæmia, as sequelæ, as each must be considered in relation to the evidence adduced in the particular case, and after exclusion, in the first two, of syphilis as a cause.
The distinction between causation and association has to be borne in mind before admitting as sequelæ of lead poisoning diseases of bacterial origin, such as phthisis or pneumonia, or any disease to which the affected person may be thought to have been rendered more prone by reason of lead employment. The contention that a person may have been debilitated by lead poisoning is no proof that the enfeeblement of the constitution was the cause either of the bacillus gaining entrance into the lung or of the ultimate fatal issue from the engrafted disease. Such assertion in every case must rest on supposition. Evidence that lead employment predisposes to phthisis is not necessarily made[61] stronger, in our opinion, by existence during life of clinical symptoms, or, in their absence, of detection of lead in the tissues post mortem.
In classifying causes of death, the general rule should be to select, from the several diseases mentioned in the certificate, the disease of the longest duration. Exceptions to this rule are that definite diseases ordinarily known as constitutional diseases should have preference over the other diseases mentioned. After thirty-five years of age, certificates of death from lead poisoning are almost always filled in in association with other diseases which are the usual causes which lead to mortality generally. But neither phthisis, nor pneumonia, nor any acute disease of the heart or lungs, nor valvular disease of the heart, nor, indeed, any acute febrile condition, can have direct relation with—i.e., be a sequela of—lead poisoning.
[1] Annual Reports of the Chief Inspector of Factories since 1898, especially for 1909, p. 19.
[2] Supplement to the Sixty-fifth Annual Report of the Registrar-General on the Mortality in Certain Occupations in the Three Years 1900, 1901, 1902, by Dr. John Tatham, pp. cxix-cxxii, Cd. 2619.
[62]
The pathology of lead poisoning has formed the subject of scientific inquiry from the time that the association of certain pathological symptoms was definitely correlated with poisoning by means of the metal or its salts.
Acute poisoning, due to accidental swallowing of large doses of lead salts or to use of lead salts criminally, generally produces a train of symptoms different from those met with in chronic industrial poisoning. But it is difficult to understand why so many writers upon the subject of lead poisoning should have attempted to draw a hard-and-fast line between the pathological symptoms in acute and chronic poisoning. This is especially the case when the after-history of cases of acute poisoning is traced, for in a large number of instances a case of acute poisoning drifts on into a subacute, and finally a chronic, stage. All the symptoms of paralysis, encephalopathy, and even kidney degeneration, have been described in persons who were first of all the subjects of acute poisoning.
The direct effect of a lead salt, such as the acetate, upon the mucous membrane of the stomach, is a caustic one, and the attention of observers seems to have been focussed on what is really a secondary effect of the lead salt, and not one intrinsically associated with actual poisoning by the metal itself.
A good deal of experimental work has also been performed in one way or another—mainly by feeding with, or inoculation of, considerable quantities of soluble lead salts—but, with one or two notable exceptions, such experiments have not carried the knowledge of the true pathology of lead poisoning very much farther. The statement is not uncommonly made that no definite correlation exists between the symptoms observed in animals and those observed in man, the reason being that the[63] massive doses given to animals cannot be similar to, nor can they produce results comparable with, the slow intoxication taking place in man; although the after-history of the majority of cases of acute poisoning shows that the symptoms suffered are generally identical with the severer symptoms seen in cases of chronic poisoning of industrial origin. One of the chief reasons explaining this remarkable point of view arises from the fact that the tissues which come into the hands of the pathologist for post-mortem and histological examination are as a rule derived from cases of chronic poisoning, cases in which the acute symptoms have drifted into the subacute or chronic stage, when any minute changes existing in the initial stages of the poisoning have long since disappeared, or their significance has been so far obscured by secondary changes that the primary lesions are lost sight of.
A critical examination of the very large amount of literature published on lead poisoning negatives the idea that acute and chronic poisoning differ fundamentally in their pathology, and observers are found describing identical pathological lesions resulting from acute or chronic industrial poisoning. It is impossible to review the whole of the existing literature. Kobert[1], in summing up the general effect of lead upon the animal body, makes the following general statement: “Lead affects especially the striped and unstriped muscles, the epithelium of the excretory glands, the neuroglia of the central nervous system, and is essentially a protoplasmic poison.”
We base our knowledge on definite experiments, so arranged that the method of exposure was in every way similar to that in lead industries. The only point of difference that can be urged against them is one of degree; but as the train of symptoms produced was in every way comparable with those suffered by man, this objection cannot be sustained. The fact that the symptoms develop in a shorter time than they do in industrial processes is merely a function of the intensity of the poisoning.
An attempt will be made first to summarize the literature on the pathology of lead poisoning, and, as such literature covers an immense amount of ground, to group the pathological findings of various observers, as far as possible, under four main headings—namely:
Gastro-intestinal system.
Nervous system.
Excretory system.
Circulatory system.
[64]
—As the chief early symptom of all types of chronic poisoning is abdominal colic, early investigators turned their attention to the pathology in this region, and ascribed the colic to various causes.
Oliver[2] noted that, in animals poisoned with lead, the intestine was found to be irregularly contracted, and ascribed the pain in abdominal colic to the irregular contraction of the intestines themselves, supposing that the effect of lead was on the muscular tissues. He also noted the presence in the intestine, both large and small, of staining due to lead, and the considerable amount of lead to be found in the large intestine. We have only met with staining by lead in the large intestine.
Dixon Mann[3] pointed out that the fæces contain two-thirds of the amount of lead taken by the mouth in experimental cases, and considered that the lead was re-excreted into the intestine; and a number of other observers hold this view. Recent work confirms the supposition, and there is no doubt that lead is eliminated in this way.
Stockvis[4] has occasionally seen small ulcers or abrasions in the small intestine; these he thinks may be due to small hæmorrhages.
Ménétrier[5], quoted by Meillère, describes a form of glandular atrophy of the stomach which is met with in chronic lead poisoning. He states that the alcoholic gastritis generally present in persons the subject of saturnine gastritis renders the differentiation exceedingly difficult. This observer is also in complete agreement with many others who associate much of the chronic lead poisoning to association with alcoholic intemperance. The particular type of gastric degeneration Ménétrier regards as due to the effect of lead is “une sclérose regulière, inter-tubulaire, se recontrant d’une manière diffuse et générale dans le muqueuse gastrique.”
He further considers that this gastric sclerosis occurs earlier than the disease of the kidneys.
Kussmaul and Meyer[6] describe a chronic intestinal catarrh with chronic degenerative changes in the intestinal mucosa very similar to the changes described by Ménétrier.
Tanquerel[7] inclined to the view that the colic was not associated with spasm of the intestines, and states that no clinical evidence could be found of intestinal spasm by rectal examination during a spasm of colic. But, as Bernard[8] points out, intestinal spasm may occur with “ballonment” of the rectum.
[65]
Another cause of colic is suggested by Bokai[9]—namely, hypersensibility of the intestinal nerves—and he cites as evidence the diminution in pain produced by the administration of morphine; and it is not improbable that some hypersensibility of the nervous system of the intestine goes hand in hand with the vaso-constriction that has been shown to exist, while, in addition, many observers have found degenerative and even subacute inflammatory changes in the sympathetic nervous system of the abdomen, mainly in the splanchnic area and the solar ganglion. The action of vaso-dilator drugs on the pain of colic demonstrates the close association of vaso-motor changes with the acute paroxysmal pain.
Riegels[10] investigated the cause of colic in 200 cases. He found that in every instance there was reason to suppose that toxic vaso-constriction of the vessels in the splanchnic area, due to irritation in the vaso-constrictor nerves, was brought about by the action of lead.
Definite gastritis has also been described in some way simulating that caused by arsenic, with thickening in the submucosa of the stomach and intestine. Associated with this enteritis is endarteritis, atrophy of the glands, and Lieberkühn’s follicles. In the colon as well as the ileum was a well-marked enteritis involving the muscular lesions of the gut.
Various other authors describe degenerative processes with cirrhotic changes in the gastro-intestinal tract. Amongst these, Galvini[11] describes, in a case of death from very chronic lead poisoning, extreme cachexia, perihepatitis, perisplenitis, atrophy of the stomach, liver, and spleen, and chronic sclerosing peritonitis (saturnine peritonitis). Associated with general changes in the abdominal cavity, marked inflammation and sclerosis was found in the solar plexus. There is said to be a very considerable correspondence between the condition of the peritoneal cavity and its contents in lead poisoning and barium poisoning. In some of the animals referred to in the description of the experiments, marked inflammatory conditions of the small intestines and colon were found, and in not a few instances definite ulceration and signs of recent hæmorrhages were found scattered along the intestinal tract. No sclerosis was found, however, in the peritoneal cavity, though a common symptom of all the animals, whether poisoned by inhalation, inoculation, or feeding, was the absence of practically all fat from the peritoneal cavity, the[66] omentum being represented by an exceedingly thin membrane without any traces of fat whatever.
—Perhaps the oldest classical symptom of lead poisoning is the potter’s palsy or wrist-drop due to interference with the nerve-supply of the extensor muscles of the hand, leading to inability to extend the wrist or the fingers on the arm, wasting of the affected extensor muscles, and finally a claw-shaped hand due to contractions produced by the pull of the unopposed flexor groups.
The origin of this extensor paralysis has been the subject of much controversy. One party regards the lesion as of central origin, affecting the upper motor neurons or their connections in the spinal cord; the other takes the view that paralysis is mainly of a peripheral type. Tanquerel[12], whose classical work on lead poisoning still contains one of the best descriptions of the disease from the clinical standpoint, describes an associated affection of the peripheral sensory nerves resulting in definite anæsthesia and hyperæsthesia, and there is no doubt that sensory nerve affection, although not very common in lead poisoning, does occur occasionally, and is due to peripheral affection of the nerves. Occasionally generalized peripheral neuritis is to be met with, but even this is much less common than in alcoholism or other toxic forms of peripheral neuritis.
In the opinion of most of the observers who regard the neuritis as of peripheral origin, the ultimate interference with the motor nerves is due to an ascending neuritis of the peripheral nerves affecting the spinal ganglia, and Pal and Mannaberg[13] have described polyneuritis; whilst Westphal[14], Dejerine[15], Eichhorst[16], Ramond[17], and others, support particularly the primary lesion of the peripheral nerves as the cause of the disease. Marie and Babinski[18] in 1894 evolved the central theory, and supported it by reference to the apparent bilateral occurrence of the paresis and the analogy with many examples of polymyelitis. Vulpian and Steiglitz[19], examining cords of animals poisoned by lead, described vacuolation of the cells in the anterior cornua of the cord.
The original suggestion of the spinal origin of the disease was enunciated by Erb[20], who, without particular reference to either the electrical or histological changes to be found in lead poisoning, based his theory on the similarity of the lesions to polymyelitis.[67] A few cords of persons who have died of lead poisoning do show slight changes in the anterior cornua.
One other theory of the nerve affections in plumbism is that advanced first of all by Hitzig[21], and later by Boerwinkel[22] and Eichhorst[23], who regard the initial disease as one related to the circulation, and not necessarily to the nerve lesions themselves. Potain[24], basing his observations on the anatomical distribution of the blood vessels, points out that the flexor muscles, excluding the supinator longus, are drained by the median cephalic vein, whilst the extensors are drained by the interosseous—a peculiarity of considerable importance, as the supinator longus escapes paralysis in the majority of cases of wrist-drop.
The presence of paralysis in the muscles and the association of nerve lesions to muscular paralysis led investigators to examine the nervous system, and probably more attention has been paid to the pathology of poisoning in this direction than in any other. Many records of isolated cases are to be found in the literature of the subject, and the examination of the spinal cord has been carried out in many instances; several have been cases of generalized paralysis or dementia, with involvement of the trunk muscles as well as those of the extremities in the paresis.
Many observers who have made histological examinations in such cases have found nuclear changes in the anterior columns of the cord suggesting polymyelitis. A small number only of the total cases of paresis, however, come under this heading. Vulpian[25], Oppenheimer[26], Oeller[27], and others, described degenerative and proliferative changes in the grey matter of the cord. Steiglitz[28] by animal experiments produced inflammatory processes in the anterior grey substance, vacuolations in the ganglion cells, and degeneration in the anterior root ganglia. In the paralyzed muscles the changes of degeneration are found: the muscle nuclei become spindle-shaped, the interstitial tissue undergoes degeneration, the muscle becomes atrophied, takes the stain badly, shows irregular striation, and the muscle bundles become ill-defined and fused.
Scarcely any two observers are agreed as to the exact nerve lesions which are to be found, and so at variance are the various theories based upon the pathological findings that it is by no means uncommon to find two sets of observers quoting the same electrical reactions and histological appearances as proving in[68] the one case peripheral, and in the other the central, origin of the paralysis. On the other hand, in the quite early observations of Hitzig[29] particular attention was called to the bloodvessels and their possible association with the disease. Moreover, as has been already cited, ancient physicians were in the habit of making use of lead as a styptic and hæmostatic because of its peculiar action on the blood.
Probably as a result of improved histological methods of examining the nervous tissue, renewed attention was given to the nerve fibres and nerve cells, with the result that, in the very large number of observations recorded, many different nerve lesions are described as the sequelæ of lead intoxication.
On the other hand, Hitzig’s observations on the associated inflammation of the bloodvessels has received confirmation by several independent workers. Westphal[30] cites a case of chronic lead poisoning resulting in death from encephalopathy, and describes degeneration and œdema of the brain following a process of chronic inflammation in the smallest and minute bloodvessels, and also associated with degeneration of the ganglion cells in the vicinity. Chvostek[31] also publishes a similar case where cerebral degeneration and some œdema had occurred. Kolisko[32], in examining the brain of a girl who had died of encephalopathy, found chronic œdema of the brain and spinal cord, the condition closely resembling that described by Hitzig as chronic cerebral hypertrophy.
Quensel[33], in a man who had died of encephalopathy, found leptomeningitis, atrophy of the cortex with degeneration of the parenchymatous elements of the cells and nerve fibres, degenerative changes in the vessels, nuclear destruction and pigmentation of the cells, and œdema. Nissl[34] described granules, which bear his name, present in the ganglion cells in the cortex, with parenchymatous degeneration. These cases were not associated with paralysis, nor is encephalopathy by any means always complicated with paralysis of muscles.
Berchthold[35] describes a case of typical spastic paraplegia due to lead, and states that the cortical neurons were but little damaged, the weight of the poison having fallen upon the peripheral segments.
Sorgo[36] describes a case of progressive spinal muscular atrophy traced to lead, in which degeneration of the spinal cord was a marked feature.
[69]
Steiglitz[37], in describing the inflammatory processes produced in animals poisoned by lead, makes special mention of a distinct minute inflammatory change in the grey matter of the brain, with vacuolation occurring in the ganglion cells in the anterior horns of the spinal cord. Prévost and Binet[38], on the other hand, describe an inflammation of the peripheral nerves occurring after administration of lead to rabbits for one month. They produced what they describe as “a lead polyneuritis,” with primary affection of the motor nerves. With the brain, as with other parts of the body, various writers describe widely differing changes. Experimentally, when the doses have been massive and the animals rapidly poisoned, very little has been found, and some regard this as evidence against the central origin of lead paresis. On the other hand, in cases of chronic poisoning (mainly chronic industrial poisoning), where an opportunity of post-mortem examination has been afforded, marked atrophic changes have been discovered both in the brain and spinal cord, in the motor nerves supplying the affected muscles, and throughout the nervous system generally, so much so that anterior polymyelitis of old origin is described, vacuolation and degeneration of the ganglion cells, and various other pathological changes associated with nerve degeneration.
On reviewing the literature, it becomes practically certain that in the old and advanced cases of poisoning lesions are invariably found in the central nervous system, while in the less advanced cases the most marked change has been found in the peripheral nerves. In only a very few instances have any observers noted the fact of hæmorrhages occurring in the nervous tissues, and one of the most important observations on this point is the careful description by Mott[39], of a fatal case of lead poisoning in an asylum, in which distinct yielding of the vessel walls with minute hæmorrhages was present in the cerebral cortex. This case is quoted in full on p. 71.
In the record of the experiments by one of us (K. W. G.) on p. 95 are described the hæmorrhages occurring in the anterior crural nerves of cats poisoned by lead. These animals showed most distinct loss of power in their hind-limbs, as was evidenced by their inability to jump, and other symptoms homologous to wrist-drop in man. In these animals no nerve degeneration or alteration in the spinal cord was found sufficiently gross to account for the paralysis, whereas the amount of pressure produced[70] by the yielding of the vessel walls on the nerve bundles and the associated exudation was evidently sufficient to cause compression, and thereby loss of function in the nerve in question.
Now, the pathological changes described by numerous observers, such as parenchymatous and interstitial changes in the brain, destruction of the anterior grey matter, and, finally, the degenerative changes in the muscle groups, the macroscopical and microscopical atrophy of the muscle bundles with the fibrillation and other changes, are none of them opposed to the view that hæmorrhage and exudation are the earliest and initial change; in fact, in experimental animals minute hæmorrhages could always be traced in the earliest stages of poisoning, frequently before definite symptoms appeared.
Confirmation of this theory is seen in the work of Glibert[40], and the drawings he gives showing fibrous changes in the lungs, hyperplasia, congestion, and emphysema, cirrhotic conditions of the liver, and, as he describes it, blood-stasis caused by elongation and dilatation of the capillaries, are all of them highly confirmatory of the hæmorrhage theory. Further confirmation is also afforded by the observations on the action of lead salts upon the blood itself. From the earliest days lead has been used as a styptic, and its empirical use has been shown by later observation to be due to its power of readily coagulating albumin and peptone. Further, in chronic lead poisoning there is a marked increase in the coagulation time of the blood. Glibert, in the work already referred to, points out the increased ductility of the red blood-cells in lead poisoning. Quite apart from this, there is no doubt that definite alterations in the red blood-cells occur; a species of icterus is common in lead poisoning, the anæmia of lead poisoning is of a destructive type, increased urobilin may occur in the urine, and in some instances hæmatoporphyrin is present in considerable quantities. The bone-marrow in cases of lead poisoning undergoes distinct inflammatory change, and may possibly be the cause of some of the curious aching arthralgias often noted as a clinical symptom. All the pathological evidence that can be adduced points unmistakably to the blood as suffering the initial stress in lead poisoning, and it is therefore by no means surprising that the blood vessels should be the next in order to undergo degenerative changes. It is probably this degenerative change, particularly associated with the increased coagulability, alteration[71] in viscosity, the destruction of the blood-cells themselves, and the permeation of the vessel walls by definite, if exceedingly minute, quantities of lead salts, that determines the yielding of the smaller and generally the weaker bloodvessels. In the histological examination of the experimental animals there was considerable evidence that the venules, rather than the arterioles, are the first to yield.
The following case of chronic lead encephalitis, with the examination of the nervous system described by Mott[41], is a case that has a large bearing on the general pathology of lead poisoning, and has the merit of being so carefully described that we cite it at length, as bringing out some of the special features connected with the pathology of lead poisoning.
The patient was a coach-painter, aged forty-four. Family history of no particular interest. Had been a painter since a boy. No specific history. Treated for enlargement of liver at one time. Married. No children. His wife a widow; four children before married to him.
Before the attack of encephalitis, which ultimately resulted in his death, he suffered from colic and obstinate constipation. The commencement of the final attack of lead infection was associated with an epileptiform fit, from which he recovered and resumed his work, but from this time onwards he suffered from progressive weakness and progressive inability to perform his ordinary work. Constant indulgence in alcohol did not pull him together as before; and although previously he had been able to indulge in large quantities of alcohol, a very little now affected him adversely.
The first epileptiform attack was in July, and in November he commenced to have delusions, was restless, suspicious.
On admission to the asylum he showed marked cachexia. Weight, 8 stone 7 pounds; height, 5 feet 9 inches. There was present well-marked oral sepsis and blue line.
Mental Condition.—Restlessness; disorientation; remitting delirious state; periods of shouting coincident with colic, worse at night; auditory hallucinations.
Physical Condition.—Bilateral wrist-drop; extensor paralysis of the fingers; hand-grip and gait impaired; reaction of degeneration of paralyzed muscles; coarse tremors; fibrillary twitching; staccato articulation.
Sensory.—No definite change.
Reflexes.—Pupils normal. Sluggish reaction to light and accommodation.
Organic.—Deglutition difficult. Micturition and defæcation not under control.
Vaso-motor.—Tâche cérébrale marked.
Eye neuro-retinitis. Unequal amaurosis.
Heart.—Increased action, variable; alteration during exacerbations of colic. Second sound in aortic area accentuated. High pressure, variable. Majority of arteries thickened.
He suffered gradual mental change; the whole of the mental symptoms increased in severity until the patient looked like the final stages of a case of general paralysis. He died on December 1. Colic was present at intervals during the whole time.
Post-mortem made the next day. Septic bronchitis. Hæmorrhage at the base of epiglottis and left vocal cord.
Lungs.—Septic broncho-pneumonia.
Pericardium.—Small amount of fluid.
Heart.—Striated, bluish. Weight 11¹⁄₄ ounces.
Ventricles.—Slight hypertrophy of left ventricle.
[72]
Valves.—Competent.
Aorta.—Atheroma near its bifurcation.
Arteries.—All more or less thickened.
Peritoneum.—Retroperitoneal hæmorrhage in region outside pancreas. Mesenteric glands enlarged, indurated, bluish on section.
Stomach.—Normal.
Intestines.—Vessels congested. Large bowel constricted at irregular intervals.
Cæcum.—Mucosa slate-coloured.
Colon.—Dark-greenish mass.
Liver.—Blue on section; pale yellow areas; soft in consistency. Weight, 47³⁄₄ ounces.
Spleen.—Normal.
Kidneys.—No fat. Cirrhotic, adherent, atrophic cortex, granular.
Muscles.—Generally dark in colour; wasted.
A very complete histological examination was made of the brain and spinal cord, and throughout the particular changes noticed were proliferation of the glia, hyaline thickening of the walls of the vessels, both arteries and veins, and presence of congestion; and here and there rupture of the smallest vessels, causing miliary microscopic hæmorrhages into the perivascular sheaths and the substance of the brain. There was no infiltration with lymphocytes and plasma cells, as is found in general paralysis. The neuroglia showed a formative hyperplasia resulting from chronic irritation.
In the cortex there was neuroglia proliferation in the polymorpho layer and the molecular layer. Changes were seen in the Betz cells, particularly in the Nissl substance, with perinuclear chromatolysis, such as is generally found in chronic peripheral neuritis, whether due to lead, alcohol, or other toxic causes.
There was no coarse atrophy or degeneration of the fibres of the cortex. Neither the cerebellum nor the spinal cord at any of the levels examined showed fibre atrophy or degeneration, except possibly a slight diffuse sclerosis in the crossed pyramidal tracts of the lumbar region.
Microscopical examination was made of the heart, spleen, kidney, liver, lung, and suprarenal gland. There was a general condition of angiosclerosis; in the liver a fibrotic overgrowth around the vessels; in the kidneys well-marked interstitial fibrosis.
A chemical examination of the brain was also conducted by the copper potassium nitrite method, but no lead was found.
—A large number of observers have shown that great stress is thrown on the kidney in the excretion of lead. Discussion has taken place as to whether the effect is a primary interstitial or a parenchymatous nephritis. Most observers are agreed that the histological changes found in the kidneys of lead workers have very little by which they may be differentiated from the effects of alcohol.
Although the kidney suffers directly from the effect of circulating lead, the amount of lead excreted by the kidney in chronic cases is usually small, variable in quantity, and very rarely exceeds more than 5 milligrammes in the twenty-four hours.
The chemical estimations of the quantity of lead found in the kidney of persons who have died of lead poisoning given by different observers, vary exceedingly. Even in cases of definite lead poisoning, where there can be no reasonable doubt as to[73] the diagnosis, many cases are on record where no lead at all has been discovered in the kidney.
It is not acute nephritis which is seen in lead poisoning, but the chronic cirrhotic variety. This probably takes a very long time to develop; indeed, animals kept under the influence of lead for two years show very little kidney destruction. It is quite possible that in the kidney disease met with in lead-workers the combined effect of alcohol with lead is really the causative factor. There is not sufficient statistical evidence to make a definite statement on this point, and it would only be possible by comparing the records of the autopsies of a number of persons working in lead who do not die from lead poisoning, and who were non-alcoholic, with a similar number of persons who, in addition to their lead absorption, were alcoholic subjects.
It is unusual to find blood in the urine, and the condition of the kidney does not suggest that it would be present.
Gull and Sutton[42] have described arterio-capillary fibrosis in which the intima of the larger vessels became greatly hypertrophied, and many of the smaller vessels are practically destroyed by obliterative arteritis. The production of arterio-sclerosis, with attendant thickening of the vessel walls and with the various symptoms commonly associated with arterio-sclerosis, were regarded as secondary symptoms of lead poisoning. The action of lead on the vessel walls themselves thus independently proved by a large number of observers working at different aspects of the problem suggests the pathological change in the vessels as the common element in the cause of these diverse symptoms of lead poisoning—colic, paralysis, mania. Generally speaking, however, the attention of most has been rather focussed on the kidney and the degenerative changes occurring in that organ due to the irritative action of lead in the process of excretion than on the vessels themselves.
Lead in the urine is by no means so common nor so definite a symptom of lead poisoning as might be supposed, considering the extreme manner in which the kidneys suffer in old-standing cases. Of particular importance in this respect is the case referred to by Zinn[43], where a woman aged thirty-three received 20 grammes of lead acetate in error. After the first acute symptoms had passed off, the case drifted on to one of chronic poisoning, with the usual symptoms of colic, anæmia, and cachexia. During the whole period of the disease, both acute and chronic[74] stages, examinations of the urine for lead were made, using the method of Fresenius Babo[44]; yet lead was only detected in the urine during the early stages, and directly the acute symptoms had passed off no further lead could be detected. This point is of some importance, particularly when taken together with the experiments quoted by Blum[45], who, injecting animals with lead iodide, was unable to recover lead from the urine. The iodide only passed through the kidney, the lead being retained in the body.
Jaksch[46] states very definitely that lead is not found in the urine in chronic cases, but only in the acute cases, and then quite early.
With regard to the kidney two views are held—the one regarding the disease of the kidney as primarily affecting the bloodvessels, and the other as an initial parenchymatous change causing secondary obstruction and alteration in the vessels themselves. There is therefore much evidence to show that, whether the bloodvessels be primarily or secondarily affected, almost all observers are in accord in the opinion that at one time or another, either sooner or later, the bloodvessels become affected through the action of lead.
Kobert[47] points out that in no case was distinct cirrhosis of the kidney produced in experimental lead poisoning in animals; inflammation certainly was to be seen, either interstitial or parenchymatous, but apparently the poisoning had not progressed a sufficient length of time for definite cirrhosis to be produced. On the other hand, kidney changes have been found of various types, all of which may be the precursors of the ultimate cirrhotic and fibroid change occurring in the kidneys seen in chronic poisoning by lead as well as in chronic alcoholism. Particular stress must be laid on the fact that cirrhotic kidneys are so frequently the direct result of long-continued alcoholic excess, and, from what has been demonstrated in the experimental researches on predisposition to lead caused by alcohol, the condition of cirrhosis of the kidney in a lead-worker is by no means indicative of lead poisoning, as it may be an old alcoholic effect long antedating that due to lead.
Oliver[48], Charcot[49], Gombault[50], Hoffer[51], and others, found a certain amount of parenchymatous degeneration. Von Leyden[52], however, was able to produce a granular condition of the kidney with glomeruli shrunken and an arterio-capillary[75] fibrosis. Gayler[53], on the other hand, thinks that the arteritis of the smallest arteries is the preliminary effect upon the kidney, whereas more recently Glibert[54] published plates of the kidney showing definite sclerosis as well as interstitial nephritis.
Cornil[55] and Brault[56] think the vessels are affected only secondarily, and that parenchymatous changes are the primary lesion. Hoffer[57], by feeding guinea-pigs with lead, produced very definite obliterative arteritis. Klemperer[58] claims to have produced inflammation and definite necrosis of portions of the kidney substance.
The whole of the kidney is not necessarily affected. Only portions of it may show changes, while Kleinenberger[59] notes that in chronic lead poisoning, at the time of acute exacerbation of the disease, granular casts as well as red blood-cells are found; and, further, that in cutting through them crystallized masses are occasionally found, consisting of urates, and sometimes containing lead.
Gayler[60] considers that the effect on the kidney commences in the muscular coats of the smaller vessels, in which endarteritis followed by obliterative arteritis is set up.
Practically all observers, therefore, are in agreement that the kidney suffers to a very considerable extent in chronic poisoning, and the majority of observers are also in agreement that the bloodvessels themselves are the primary seat of the change. Further, the presence of blood in the urine is exceedingly rare in chronic lead poisoning, despite Kleinenberger’s[61] statement to the contrary. It certainly may occur during a very acute attack, but we have never seen this symptom.
—Arterio-sclerosis occurring in lead-workers has been known for some time, and the anæmia of saturnism has been known for an even longer period. For some time no definite type of anæmia was associated with lead cachexia, and the anæmia was generally regarded as one arising from general malnutrition. Here and there through the literature of the pathology of lead poisoning are to be found remarks which suggest that the action upon the bloodvessels may be a primary instead of a secondary effect. Obliterative arteritis is described by Uhthoff[62], Pflueger[63], Oeller[64], and Pal[65], and in other cases obliterative retinitis has been considered to be associated with the action of the lead upon the vessel walls.
Heubel[66], and later Rosenstein[67], found that, in dogs[76] poisoned with lead, definite cerebral anæmia was produced, due to vaso-constriction, and consider it to be due to the direct action of lead upon the intima of the vessel walls. Associated with such poisoning were symptoms of eclampsia and uræmia, and the latter author considers that the uræmia is due to vaso-constriction of the kidney vessels.
Oliver[68] and others have also pointed out the alteration in the pulse-rate associated with exacerbations of colic, and a number of observers have noted that certain drugs, such as atropin and amyl nitrite, which are known vaso-dilators, have a distinctly calming effect upon the paroxysms of pain.
One or two other observers have actually noted the presence of hæmorrhages in the lesions; thus, in the case quoted by Mott[69] definite yielding of the vessel walls and signs of old hæmorrhage are described amongst other lesions in the brain. Seifert[70] also describes the presence of hæmorrhages amongst the ganglion cells in the anterior columns of the cord, both in the case of persons who have died of lead poisoning and animals to which lead had been given experimentally. In addition, Sajous[71] describes a case of paralysis of the superior laryngeal nerve, associated with hæmorrhages, in the region of the abductor muscles of the larynx. Mott’s case also showed this laryngeal hæmorrhage.
More recently Elschnig[72], in his observations upon the eye, has determined a close association between vaso-motor affections, constrictions, and dilatations, and various eye lesions, such as amaurosis and amblyopia, occurring in lead poisoning. Rambousek[73], in summing up Elschnig’s work, points out how much his observations tend to bridge over the gap between the action of lead upon the blood, the bloodvessels, and the nerves. He points out that the eye is a peculiarly favourable organ for watching the effect of a poison so insidious as lead. The bloodvessels, the nerves, and the muscles, are all open to inspection and actual observation to a degree not to be found in any other part of the body. Elschnig[74], in a typical case of sudden lead amaurosis associated with acute lead colic, found that very definite motor spasm of the vessels of the eye, conjunctiva, and the retina, were associated with the amaurosis. He argues from this that the action of lead is probably directly upon the unstriped muscular fibre of the vessel walls; that such an action may, and does, extend to the vessels of the eye muscles, producing[77] paralysis of the muscles of accommodation, and a dilatation of the pupil, which may be observed in a large number of persons employed in conditions subjecting them to lead absorption. Elschnig further considers that the transitory amaurosis which is often associated with lead poisoning may be due to vaso-motor disturbances in the brain itself, as well as in the eye.
Still more recently, and due, no doubt, to a great extent to the work of Elschnig, further attention has been drawn to the vascular system in lead poisoning. Elschnig’s work has carried the question another stage forward by showing the association of vaso-motor disturbances with eye disease, whilst in this country Oliver[75] pointed out the effect upon the pulse of abdominal colic.
At the beginning of the researches on this point described in the next chapter, this clue running through the whole of the pathology of lead poisoning was not appreciated. At the commencement of the investigations there seemed to be no main general line of symptoms or histological findings that could be adduced as characteristic of lead poisoning; in fact, the initial experiments were performed, with the object of examining the association of lung-absorbed lead compounds as a possible cause of lead poisoning, as against the entrance of lead by the alimentary canal; but as the experiments proceeded it became clear that the stress of the initial intoxication was undoubtedly falling upon the bloodvessels, and more particularly upon the minuter bloodvessels, and less on the arterial side of the capillaries (although the capillaries were to a large extent associated with the process) than upon the venous radicles.
A general consideration of the pathology shows that lead causes changes in the nervous system affecting both upper and lower segments, degeneration of the ganglion cells in the cord and in the brain, interstitial inflammation of the neuroglia, cortical degeneration, distinct neuritis, both axial and peri-axial, of the peripheral nerves, and also signs of change in the sympathetic nervous system in chronic lead poisoning. Later work has, however, all tended to point out that the chief and first effect of lead is upon the blood.
Moritz[76] first pointed out the presence of basophile granules in the red blood-cells. The work was followed up by Emden[77], Gravitz[78], Zinn[79], Otto[80], Silbert[81], and by Escherich[82]. All these authors found basophilic erythrocytes in the[78] blood associated with blood-destruction, and Escherich in addition describes early changes taking place in the intima of the bloodvessels associated with vaso-constriction. The Italian author Mattirolo[83], as well as Marchet[84] and Jores[85], came to a similar conclusion. Glibert of Brussels[86], carrying the observations somewhat farther, and although working with guinea-pigs, which normally show basophilic staining in their blood-cells, was able to demonstrate one further point of considerable value—namely, the increase in the viscosity of the blood, with blood-corpuscles of greater toughness, elasticity, and power to resist destruction when making films, than in normal blood.
There is thus very striking continuity in the observations of all the various observers, despite the fact that at first sight their descriptions may appear discordant. There seems no doubt that practically all authors who have given attention to the subject are agreed that the circulatory system, and primarily the blood circulating in the vessels, is affected by lead, and, further, that the vessels themselves undergo degeneration of various types, many of the cases examined showing complete obliterative arteritis as the result of long-standing irritation. Others describe no obliterative changes of this type in the vessels, because attention was given mainly to the nervous system, where the cells were found degenerated and showing chromatolysis. But, on the other hand, careful observers, such as Mott, have noted the presence, in passing, of these apparent yieldings of the vessels here and there in the region of the degenerated nervous tissue. Again, even the histological action of a drug such as amyl nitrite points to involvement of the vaso-motor system. Perhaps this curious association through all the described pathology and bloodvessel infection would not appear so clear but for the more recent investigations described in the following chapter.
[1] Kobert: Lehrbuch der Intoxikationen, 2te Aufl., 1906, p. 361.
[2] Oliver, Sir T.: Lead Poisoning. 1891.
[3] Dixon Mann: Forensic Medicine and Toxicology, p. 495.
[4] Stockvis: International Congress of Industrial Hygiene. Brussels, 1910.
[5] Ménétrier: Meillère’s Le Saturnisme, pp. 131-136.
[6] Kussmaul and Meyer: Deutsches Archiv für Klin. Med., ix., p. 283.
[7] Tanquerel: Traité des Maladies de Plomb, ou Saturnines. Paris, 1839.
[8] Bernard: Meillère’s Le Saturnisme, p. 155.
[9] Bokai: Trib. Med., June 11, 1891.
[10] Riegels: Kobert’s Lehrbuch der Intoxikationen, p. 363.
[79]
[11] Galvini: Rivista Clinica, fasc. iii., 1884.
[12] Tanquerel: Ibid.
[13] Pal and Mannaberg: Revue Générale de Villaret, Gaz. des Hôp., Fév. 16 and 19, 1903.
[14] Westphal: Archiv f. Phys. u. Nervenkr., 1874.
[15] Dejerine: Mém. de la Soc. de Biologie, 1879, et Exposé de Titres, p. 58, 1894.
[16] Eichhorst: Ueber Bleilähmung. Virchow’s Archiv, 1890, p. 217.
[17] Ramond: Maladies du Système Nerveux, t. xi. 1895, 1896.
[18] Marie and Babinski: Meillère’s Le Saturnisme, p. 193.
[19] Vulpian and Steiglitz: Archiv für Psych., 1892, xxiv., p. 1.
[20] Erb: Berl. Klin. Woch., 1884, p. 110.
[21] Hitzig: Studien über Bleiverg. Berlin, 1868.
[22] Boerwinkel: Virchow’s Archiv, Bd. cxx., 1890.
[23] Eichhorst: Ibid.
[24] Potain: Bull. Med., 1887.
[25] Vulpian: Maladies du Système Nerveux. 1879.
[26] Oppenheimer: Zur Kennt. der Exp. Bleiverg. Berlin, 1898.
[27] Oeller: Path. Anatom. der Bleilähmung. München, 1883.
[28] Steiglitz: Archiv für Psychiatrie, Bd. xxiv., 1892.
[29] Hitzig: Ibid.
[30] Westphal: Archiv für Psychiatrie, Bd. xix., 1888.
[31] Chvostek: Neurol. Centralblatt, 1897.
[32] Kolisko: Die Bekämpfung der Bleigefahr in der Industrie, von Leymann, p. 21. 1908.
[33] Quensel: Archiv für Psychiatrie, Bd. xxxv., 1902.
[34] Nissl: Allgemeine Zeitschrift für Psychiatrie, Bd. xlv., 1892; Bd. iv. 1897.
[35] Berchthold: Die Bekämpfung der Bleigefahr in der Industrie, von Leymann, p. 23. 1908.
[36] Sorgo: Wien. Med. Woch., 1900.
[37] Steiglitz: Archiv für Psychiatrie, Bd. xxiv., 1892.
[38] Prévost and Binet: Revue Médicale de la Suisse Romande, ii., 1889.
[39] Mott: Archives of Neurology and Psychiatry, vol. iv., p. 117.
[40] Glibert: Le Saturnisme Expérimental: Extrait des Rapports Ann. de l’Insp. du Travail, 1906.
[41] Mott: Ibid.
[42] Gull and Sutton: Kobert’s Lehrbuch der Intoxikationen, 2te Aufl., 1906, p. 370.
[43] Zinn: Berl. Med. Woch., 1899.
[44] Fresenius Babo: Liebig’s Annalen, vol. xlix., p. 287. 1844.
[45] Blum: Wien. Med. Woch., No. 13, 1904.
[46] Jaksch: Klinische Diagnostik.
[47] Kobert: Ibid., p. 369, and general Literature, p. 376.
[48] Oliver, Sir T.: Lead Poisoning. 1891.
[49] Charcot: Leçons sur les Maladies du Foie et des Reins. Paris, 1882.
[50] Gombault: Archiv für Physiologie, 1881.
[51] Hoffer: Dissertation, Freiburg, 1883.
[52] Von Leyden: Zeit. für Klin. Med., 1883.
[53] Gayler: Ziegler’s Beitr., ii., 1888.
[54] Glibert: Ibid.
[55] Cornil: Journal de l’anat. et physiol., No. 2, 1883.
[56] Brault: Loc. cit.
[57] Hoffer: Loc. cit.
[58] Klemperer: Kobert’s Lehrbuch der Intoxikationen, 2te Aufl., 1906, p. 370.
[59] Kleinenberger: Münch. Med. Woch., No. 8, 1904.
[60] Gayler: Loc. cit.
[61] Kleinenberger: Loc. cit.
[62] Uhthoff: Handbuch der Aug. Lief. Leipzig, 1901.
[63] Pflueger: Die Bekämpfung der Bleigefahr in der Industrie, von Leymann, p. 21. 1908.
[64] Oeller: Ibid.
[80]
[65] Pal: Zentralbl. f. innere Med. Leipzig, 1903.
[66] Heubel: Path. und Symp. Chron. Bleiverg. Berlin, 1871.
[67] Rosenstein: Virchow’s Archiv. 1897.
[68] Oliver, Sir T.: Lead Poisoning. 1891.
[69] Mott: Ibid.
[70] Seifert: Berl. Klin. Woch., 1884.
[71] Sajous: Archiv für Laryng., iii., 1882.
[72] Elschnig: Wien. Med. Woch., 1898.
[73] Rambousek: Die Bekämpfung der Bleigefahr, von Leymann, p. 15. 1908.
[74] Elschnig: Loc. cit.
[75] Oliver, Sir T.: Lead Poisoning. 1891.
[76] Moritz: St. Petersb. Med. Woch., 1901.
[77] Emden: Die Bekämpfung der Bleigefahr, von Leymann, p. 19.
[78] Gravitz: Deutsche Med. Woch., No. 36. 1899.
[79] Zinn: Berl. Klin. Woch., 1899.
[80] Otto: Revue Méd., 1892.
[81] Silbert: Ibid.
[82] Escherich: Die Bekämpfung der Bleigefahr, von Leymann, p. 18.
[83] Mattirolo: Ibid., p. 19.
[84] Marchet: Ibid., p. 19.
[85] Jores: Ziegler’s Beitr., Bd. xxxi., 1902.
[86] Glibert: Ibid.
[81]
[A] This chapter is the work entirely of one of us (K. W. G.)
It was thought that some light might be thrown on chronic intoxication produced by lead salts if direct experiment were made upon animals, resembling in the arrangement of such experiments, as far as possible, the industrial conditions under which human beings contract lead poisoning.
The animals chosen for the experiments were cats, as it is a fact of common knowledge that it is impossible to keep cats in lead works, particularly white-lead, because they rapidly become poisoned if allowed to stray about the works. The same holds good in the case of dogs.
From the statistics already given in Chapter IV., and from the remarks in the chapter on Ætiology, there was no doubt whatever that dust played a most important rôle in the production of industrial lead poisoning. In attempting, therefore, to copy the industrial conditions, it is essential to submit the animals experimented upon to infection by means of air in which lead dust is suspended. A large number of experiments have been carried out in the first place, by myself[1], and later in conjunction with Dr. Goodbody[2], and another series of experiments were also undertaken by myself[3]. Further experiments are still in progress in this and other directions.
—First Series.—A. The animals experimented upon were placed in a large closed chamber at one end of which an electric fan was fitted in such a way that the air was kept in constant motion. The lead dust was introduced by means of a funnel through the roof in a definite quantity during timed intervals. By means of an aspirating jar and[82] a tube inserted into the side, samples of air were withdrawn from time to time during the experiments, and submitted to chemical analysis to determine the quantity of lead circulating in the air. These samples were drawn off at the level of the animals’ heads. Great care was taken to eliminate any swallowing of dust by the animals during the experiments, by protecting their coats from the dust and carefully brushing them at the conclusion of each exposure.
Second Series.—B. In other experiments a chamber containing two separate compartments was constructed, and lead dust suspended in air was blown into the two compartments by means of an electric fan situated outside. The apparatus was so arranged that the draught of air from the fan passed through two separate boxes, in which the lead compound under experiment was kept agitated by means of small fans situated in the boxes and driven by a second electric motor. In this way two different samples of lead were experimented on at one and the same time, the air current driving in the dust through the two boxes being equal on the two sides; the quantity of dust was therefore directly proportional to the compound used. Samples of the dusty air were aspirated off and subjected to analysis, as in the first series. In this series of experiments the animals were so arranged that only their heads projected into the dust chamber during exposure.
—Feeding experiments were carried out by mixing a weighed dose of the lead compound experimented with, and adding this to a small portion of the animal’s first feed in the morning. It was found that unless the lead was well incorporated with the animal’s food it would not take the lead in the dry form; and in dealing with white lead and other dust, it was necessary to give the compound in a similar form to that in which a man would obtain it under industrial conditions, which of course precluded the use of a solution.
The amount of lead given by the mouth as a control to the inhalation experiments was from seven to ten times the dose which could be taken by the animal during its exposure in the cage, and the dose was given daily, and not every third day as in the inhalation experiments. All the compounds used in the inhalation experiments were given to animals by the mouth, the animals’ weights being carefully noted.
In a further series of feeding experiments a soluble salt of[83] lead was added to the animals’ food (water or milk), the salt in this case being the nitrate. The quantity added was much smaller than in the dust experiments. 0·1 gramme was given daily.
—As a further control to both the breathing and feeding experiments, the various lead compounds similar to those used in the other experiments were inoculated into animals. The insoluble salts gave some difficulty in the technique of injection, but by using a large needle, and making the suspension of the material in the syringe, the difficulty was overcome. The quantity of material inoculated varied; it was calculated in fractions of a gramme per kilogramme body weight, the quantity of fluid used being the same in all cases—namely, 10 c.c.—and inoculations were made subcutaneously and intramuscularly in the muscles of the back after previous shaving. In only one case was localized inflammation produced, and this was when the acetate was the salt employed.
None of the animals exhibited any signs of discomfort during the experiments from the presence of lead dust in the air; once or twice sneezing was noticed, but this was an uncommon occurrence. This point is of practical importance, as the lead dust contained in the air in white-lead and other factories is not of itself irritating to the mucous membrane of the lung. The animals subjected to this form of experiment were no doubt absorbing much larger doses of lead than are persons engaged in the manufacture of lead compounds.
The only ascertainable difference in the ultimate pathological lesions produced in animals, whether inhaling large quantities or minimal quantities of dust, was the rate at which the poisoning took place. In certain experiments it was found that the animals maintained a kind of equilibrium, much as do workmen engaged in dusty lead processes. It was found, moreover, that some animals showed a certain amount of tolerance to the effect of lead dust, in that their weights remained almost constant, but an increase in the quantity of lead present in the air immediately produced progressive diminution in the body weight; and as this diminution in the body weight approached to one-third of the animal’s initial weight, so symptoms of chronic poisoning supervened.
In addition to the animals inhaling lead dust over prolonged periods, certain other inhalation experiments were made for the determination of lead dust in the lung as opposed[84] to the stomach. In the inhalation experiments proper, where the animals were exposed to inhalation every other day or every third day, for only an hour at a time, the quantity of lead present in the air was not very large, and it was thought essential to determine if, in exceedingly dusty atmospheres, any appreciable amount of lead could be found in the stomach. Ten animals were submitted to the inhalation of air heavily charged with various types of lead dust. The animal was exposed to the dust for an hour and a half to two hours; at the end of this time it was anæsthetized, and when the respirations had ceased, and the animal was dead, sulphuretted hydrogen was blown into the lung and into the stomach. The animal was then rapidly dissected and staining looked for. The tissues were further treated with acid and re-exposed to sulphuretted hydrogen gas. In one animal only out of the ten was any staining noticed in the stomach. In none of the others was any such staining found, but very definite blackening was found in the larynx, trachea, and macroscopically even in some of the bronchioles. Sections of the lung were further submitted to histological examination, and by means of micro-chemical tests with chromic acid and with iodine, and also by comparing sections of the experimental animals and animals which had not been subjected to lead dust inhalations, a very much larger quantity of material was found present in the lungs of the inhalation animals than in the normal animal. The dust was situated in the alveoli and the alveolar cells, and often in the lymphatics. On examining microscopically sections of the lungs of those animals exposed to graduated inhalation over extensive periods, a far larger number of blackened granules, dust, pigment, and other substances, was found than in similar sections of animals which were under normal conditions and had not been exposed to lead dust, although it is true such animals show a very fair proportion of carbon particles taken up by the lung tissue.
A further important fact was noticeable in animals Nos. 21 and 22 (see p. 101), which had been exposed to a low solubility glaze such as is used in the Potteries. Low solubility glaze is compounded with lead frit—that is to say, a lead glaze (or lead silicate) which has been finely ground. The particles of this substance are much larger than those of ordinary white lead, and in addition they are much more angular. Of three animals exposed to this glaze, one actually died of pneumonia (acute),[85] and the other two suffered from some bronchial trouble, both of them showing distinct signs of pneumonic patches and old and chronic inflammation when examined histologically; whereas in none of the other animals exposed to white lead dust or to the high solubility glaze, which contained white lead as opposed to lead frit, no such pneumonic or fibroid changes were found. This point is of some pathological importance.
The inhalation experiments also throw some light on the quantity of lead necessary to produce poisoning. The animals in the inhalation experiments were exposed for varying periods, and constant estimations made of the lead present in the air. In a number of instances samples were taken from the cage air during the whole of the experiment, as rapidly as possible. The quantity of lead floating in the air was found to increase as the experiment progressed, although a large amount of the lead introduced was caught on the side of the cage and deposited on the floor.
In the later experiments the method of taking the samples continuously during the experiment was abandoned, and four samples only were taken, and the average recorded. A simple calculation will give the quantity of lead dust it would be possible for an animal to inhale during the whole of this period of exposure. The utmost tidal air in the case of a cat would be taken at 50 c.c. Taking the average, about 0·27 gramme of lead was inhaled during the half-hour of exposure.
—Twelve feeding experiments of various types are recorded. The method of experiment was as follows:
The compound under investigation was carefully weighed out each day (0·5 to 1·0 gramme), the substance being some of the same compound that was being made use of for inhalation experiments. In the case of the white lead it was found essential to mix it with the animals’ food; they were given white lead in a small amount of their food, and no further food was given for some little time after the dose of lead had been swallowed. Low and high solubility glazes were also made use of for feeding, and as a further experiment alcohol was given to the animals in addition to the previous course of lead inhalation or feeding, and the exposure to lead continued after the alcohol was given. In addition to high solubility glazes, white lead, and flue dust, a soluble salt of lead was also used, in one series the salt being the nitrate. 0·1 gramme was given daily; and it is[86] these two nitrate animals (46 and 47) which showed distinct differences from the white lead and other feeding experiments. In one case the lead was given mixed with water, in the other mixed with milk. The animal which was fed with the nitrate dissolved in water developed encephalopathy, whereas the one in which the substance was mixed in milk exhibited no signs, though fed for a similar period. Both the animals increased in weight, which is an unusual effect in experimental lead poisoning. The question of the addition of milk, which apparently prevented the absorption of lead, is of very considerable importance, as it is highly probable, as has been pointed out with regard to the precipitation of lead by means of organic substances, that the albuminoid substances in the milk precipitate the nitrate already in a state of solution; and it may be argued from these experiments that mixing the white lead with the food would tend to prevent the lead having a toxic or deleterious effect, but even when the lead was given in the form of pills between meals no poisonous effect was noticed. Further, the quantity of white lead given was considerable, and it is highly questionable whether the quantity of lead so taken would be dissolved by the gastric juice excreted under normal circumstances in its entirety, as a very considerable quantity would pass onwards through the pylorus undissolved. Until the lead compound has become soluble it cannot react with the albuminoid constituent of the food. Ordinary dry white lead or litharge does not combine directly with albumin.
The majority of the experimental animals showed alteration in weight. The most important point which is brought out by these experiments, considering them from the point of view of inhalation, is the enormous quantity of white lead the “feeding” animals swallowed without producing any apparent symptoms. The quantities cited are the amounts given per diem, whereas in the inhalation experiments the animals were rarely exposed more than three days a week for an hour at a time (see table, p. 101). The quantity of lead, therefore, given by the gastro-intestinal canal was at least ten times as much, in many cases fifteen or twenty times as much, as could be taken by the other animals via the lung during inhalation, and yet these animals showed little or no susceptibility to poisoning when fed with white lead or other lead compounds, unless alcohol was given in addition.
[87]
An examination of the stomach after death showed, in the case of the alcoholic animals, distinct evidence of gastritis, and there is some reason to suppose that in such animals a degree of hyperacidity may have existed, thereby promoting the rate of solution of the lead.
The increased susceptibility to lead poisoning through the agency of alcohol is interesting. No. 6 received, in addition to its inhalations or period of exposure in the dusty air, 50 c.c. of port wine per diem. Symptoms of lead poisoning appeared a day sooner than in any other animal, and if we eliminate this experiment, as the dust (flue dust from blast-furnace) contained also arsenic and antimony, three days sooner than the litharge animal, and twenty-five days sooner than the other animals exposed to white lead dust. In addition, this animal was the only one which actually died during the period of experiment; all the other animals were killed at the end of two months and submitted to histological examination; but the animal which had received alcohol died with symptoms closely simulating lead encephalopathy in man. The predisposing action of alcohol is still further emphasized by the subsequent experiments with three animals exposed to white lead dust; one was exposed thirty-seven and the other thirty days before symptoms appeared, whereas when alcohol was given poisoning was apparent within twelve days, and after only four inhalations.
In the case of animals fed with white lead, one after eight months, and the other a year and a half, showed no signs of lead poisoning at all, while the weights remained constant. At the end of this time alcohol (50 c.c. of port wine) was added to the animals’ diet, and one month after the addition of alcohol to the diet, the dose of white lead being continued constant, encephalopathy ensued. In a second case the animal was started on alcohol in addition to the white lead. In a month it was showing signs of slight paresis. Again, an animal fed on a low-solubility frit consisting of ground-up lead silicate, showed no ill-effects after receiving a daily dose of this compound. At the end of this time alcohol was added to its diet, and six months later the animal developed symptoms of cerebral involvement, which continued at intervals until a fatal attack of encephalopathy at the end of a year. There is thus definite evidence to show that the addition of alcohol to the animal’s diet undoubtedly hastened and determined the appearance of[88] lead poisoning, and this, taken in conjunction with the inhalation experiments previously cited, is very strong evidence of the increased susceptibility to lead poisoning produced by alcohol. This supersensitiveness to lead through the medium of alcohol is a matter of clinical experience to most persons who have had experience of industrial lead poisoning, particularly those who have been engaged in the routine examination of persons working in factories.
—In order to control both the feeding and the inhalation experiments, and more particularly to obtain direct information of the effect of lead upon the body tissues, resource was had to the inoculation of the various lead compounds tested—namely: (1) White lead, (2) litharge, (3) lead frit. These three compounds are the three types of lead salt which are used in the Potteries, whilst white lead and litharge are the compounds causing industrial poisoning in the largest proportion of cases in other industries. As a further control, the more soluble lead salts were also made use of—namely, acetate, nitrate, and chloride—mainly for the purpose of establishing some standard of poisoning both in rate and dose.
Several rather unexpected results were derived from the inoculation experiments, which will be referred to.
The method of inoculation was to suspend the lead compound to be tested in normal saline solution or distilled water. The animal was then shaved, and the lead compound inoculated into the muscles of the back. The corrosive action of these lead salts was avoided by using a considerable quantity of diluent.
Lead frit is a constituent of low-solubility glaze—that is to say, a glaze which has not more than 5 per cent. soluble lead when subjected to the standard test of exposing 1 gramme of the glaze to a litre of 0·04 per cent. hydrochloric acid for an hour at room temperature. The frit which was the constituent of this glaze is produced by heating together litharge or lead and silica, the production being a yellow, hard, glaze-like material looking very much like sugar-candy. It is not by any means a compound of lead and silica of simple composition, as different samples show a wide variation in their lead content; whilst, in addition, the mode of its formation closely resembles that of an alloy or amalgam, and allows of the formation of a eutectic entangling in its meshes both of the constituents of which it is formed, so that a certain amount of free lead, in addition to the[89] silicates of various descriptions, are present. At the same time the compound is highly resistant to the action of mineral acids, and, of course, much more insoluble and refractory than white lead, litharge, or other lead oxides. The body fluids, however, particularly the fluids in the subcutaneous and muscular tissue, definitely exert some action upon this fritted lead, and it was found experimentally that symptoms of poisoning could be produced in the experimental animals when even small doses were administered. A gramme of frit was inoculated, and in all but one case the animals showed definite signs of lead poisoning, and in two instances actually died with symptoms of encephalitis.
By washing the frit with distilled water, a slight diminution in the poisonousness was found, but by washing the frit with two or three changes of dilute acetic acid (3 per cent.), and then with distilled water, no pathological results followed inoculation. Water-washing frit alone definitely reduces the poisonous effect, but not to the same extent as the preliminary washing with acetic acid. On the other hand, washing with hot water had a much greater effect than cold-water washing.
Further evidence given by the inoculation experiments shows the relationship between the more soluble and the insoluble lead salts. The dose of acetate required to kill an animal was about 0·1 gramme of acetate per kilogramme body weight. On the other hand, 0·1 gramme of white lead produced no ill-effects, 0·5 gramme per kilogramme body weight produced death in about two months. In addition, those animals suffering from the more acute forms of poisoning developed definite eye changes and retinal hæmorrhages. Tortuosity and increased size of the retinal vessels were observed in several instances.
Besides controlling the experiments of feeding and inhalation, the inoculation experiments play a still more important part, as they furnish the correlation, necessarily, of the histological changes found as the result of poisoning by means of lead. In all the animals which have died of poisoning, certain definite trains of symptoms made their appearance. These symptoms were in practically all particulars similar to those observed in industrial lead poisoning in man, the onset of the affection and its clinical course corresponding to the symptom-complex in man, including those of cortical involvement, and often similar to the classical Jacksonian variety.
[90]
Throughout these experiments the animals exhibited no signs of irritation, and during the initial period, even, when loss of weight was a noticeable feature, their appetites remained exceedingly good; they were quite friendly, and purred loudly when stroked; but when symptoms of poisoning became manifest, particularly the onset of paralysis, a definite change in mental phenomena took place: the animals became quarrelsome, highly apprehensive of danger without cause, morose and lethargic by turns. At this stage, in more than one instance, acute encephalopathy supervened. The mental change was peculiarly striking in reference to Mott’s case, quoted on p. 71, as in all respects it was exactly analogous with the train of symptoms recorded in that case. To sum up, the symptoms produced in the experimental animals by the lead compounds inoculated and respired, no matter what the particular compound of lead experimented was, were as follows:
1. Slight preliminary rise in weight at the commencement of the experiment, lasting from one to two weeks.
2. Progressive loss of weight, mainly due to the disappearance of all fat, subcutaneous, kidney, mesenteric, etc., with associated anæmia, and the curious sunken and pinched faces commonly associated with saturnine cachexia.
3. Paresis of various types.
In the cat the muscles first affected are those of the back and the quadriceps extensor of the hind-limbs. The onset of the paralysis is slow and insidious, but may be acute; as a rule weakness in the muscles of the lumbar region and the spine are the first symptoms; secondly, inability to jump, owing to the weakness of the quadriceps extensor, while the animal tends to fall over when turning round quickly. Encephalitis occurs, and is frequently fatal. As a rule the affection is unilateral; complete loss of consciousness may occur, followed by slow but complete recovery. The animals gave no evidence of suffering pain, and, when recovered from an attack of encephalopathy, would at once take milk, but seemed dazed and uncertain in their movements. When the animals reached the stage of paralysis, they were destroyed under anæsthetics, and subjected to post-mortem examination. The post-mortem findings of a typical case were as follows:
The animal was emaciated, the fur easily pulled out, and the muscles were exceedingly flaccid.
[91]
Rigor mortis was slow in making its appearance; the blood remained fluid for a considerable time.
Practically no fat was to be found in the whole of the mesentery, and the omentum was devoid of fat and shrivelled. The fat around the kidneys had entirely disappeared. There was little orbital fat.
The peritoneum was thin and glistening, and very frail.
The whole of the mesenteric vessels, particularly in the region of the large intestine and the ileo-cæcal valve, were engorged with blood; whilst in the lower part of the small intestine, and often in the duodenum, occasionally in the whole of the jejunum and ileum, traces of minute hæmorrhages were found along the intestinal wall.
The liver was engorged with blood, as was the spleen.
The kidney capsule stripped easily, but was occasionally adherent here and there. The whole of the cortical vessels were injected with blood, the branching showing most distinctly.
A good deal of serous fluid was at times found underneath the kidney capsule.
On section the cortex appeared engorged with blood, and showed here and there, even to the naked eye, small hæmorrhages.
In the region of the appendix a few large mesenteric glands were invariably found, whilst a few glands might also be found in the wasted mesentery of the small intestine. In the region of the appendix the glands were frequently dark in colour. On opening the gut, minute hæmorrhages and ulcerated patches were to be found in the lower part of the ileum; the ileo-cæcal valve, and the whole of the large intestine, extending right up to the end of the appendix, was covered with a dark slate-blue slime, in which lead could be easily recognized by chemical processes.
Ulceration of the gastric mucous is uncommon, and only on one occasion were any hæmorrhages found. In the thoracic cavity the lungs were generally found to be emphysematous, and particularly in those animals subjected to inhalation of lead frit containing angular particles of lead glaze broncho-pneumonia was found.
The heart was flabby, and occasionally distinct roughening and thickening of the valves was seen.
Nervous System.—On opening the skull, hæmorrhages were[92] frequently found at the base of the brain, occasionally situated over the surface of the cerebrum. Minute hæmorrhages were found often underneath the arachnoid membrane, but the largest hæmorrhages were always found at the base of the brain, and spreading down into the spinal canal along the medulla.
On removing the cord, minute hæmorrhages were found along the surface, irregular in distribution, and never very large. On section the brain and cord appeared normal.
—A large number of sections were prepared from the animals developing symptoms of poisoning; the various tissues are described seriatim:
Muscles.—These appear to have undergone general fatty degeneration. The individual muscle fibres are indistinct in outline, and show irregular areas stained by hæmatoxylin. Some infiltration may be seen here and there between the muscle fibres, and minute hæmorrhages are occasionally detected, the chief appearance being that of general atrophy. The heart muscle shows similar degeneration, and the tendency of the sarcolemma to break down and stain irregularly is apparent. In many areas the muscle fibres stain poorly, if at all. Occasionally minute hæmorrhages are found, passing between the muscle fibres.
Liver.—The hepatic cells show varied degeneration; the vessels passing between the cells are engorged with blood, the cells being frequently much distorted from their general arrangement, and here and there completely obliterated by small areas of exudation as well as actual hæmorrhages.
Spleen.—The parenchyma shows masses of irregular spaces filled with recently-shed blood; the individual cells show a granular degeneration, with occasional basophile staining, the general appearance being one of chronic congestion. Here and there cloudy swelling may be seen.
Intestine.—Sections across the small intestine show atrophy of the intestinal wall, slight degeneration of the muscular coats, with infiltration and minute hæmorrhages.
Large Intestine.—Here similar minute hæmorrhages are found, in no case large enough to be seen by the naked eye. Areas of necrotic tissue are also seen in which considerable quantities of lead sulphide particles are found.
PLATE I
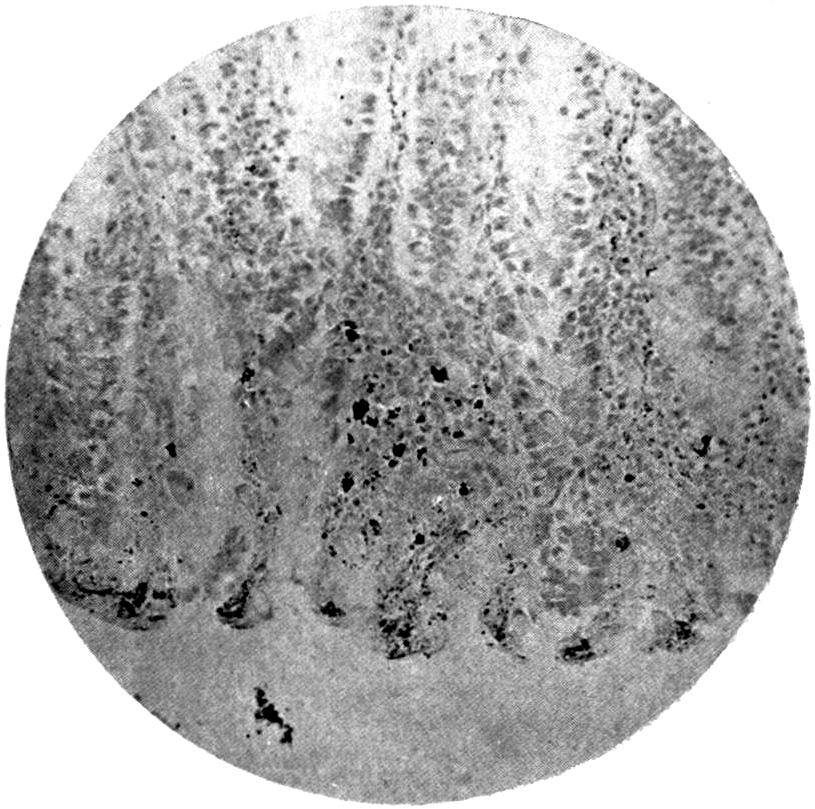
Fig. 1.—Section of Large Intestine of Animal poisoned by Inhalation of White Lead, showing Excretion of Lead by Tissues. (Stained Eosin and Hæmatoxylin.) × 250.
The whole of the large intestine was stained black, the staining commencing at the ileo-cæcal valve. No staining is observable in the small intestine; the line of demarcation is sharp.
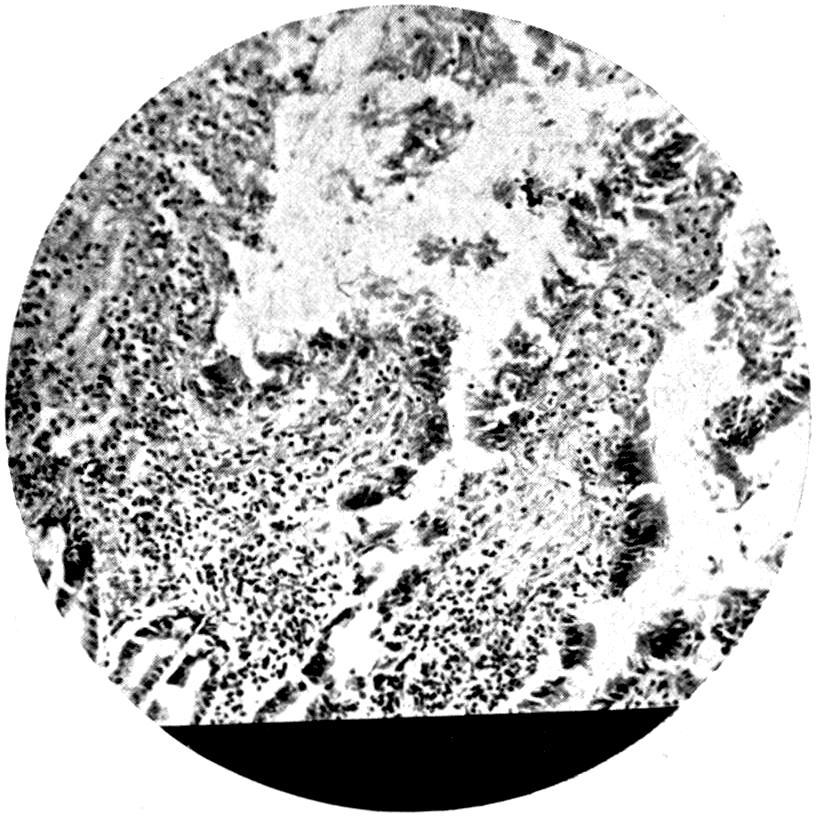
Fig. 2.—Intestinal Ulceration in Turpentine Poisoning. (Stained Eosin and Hæmatoxylin.) × 250.
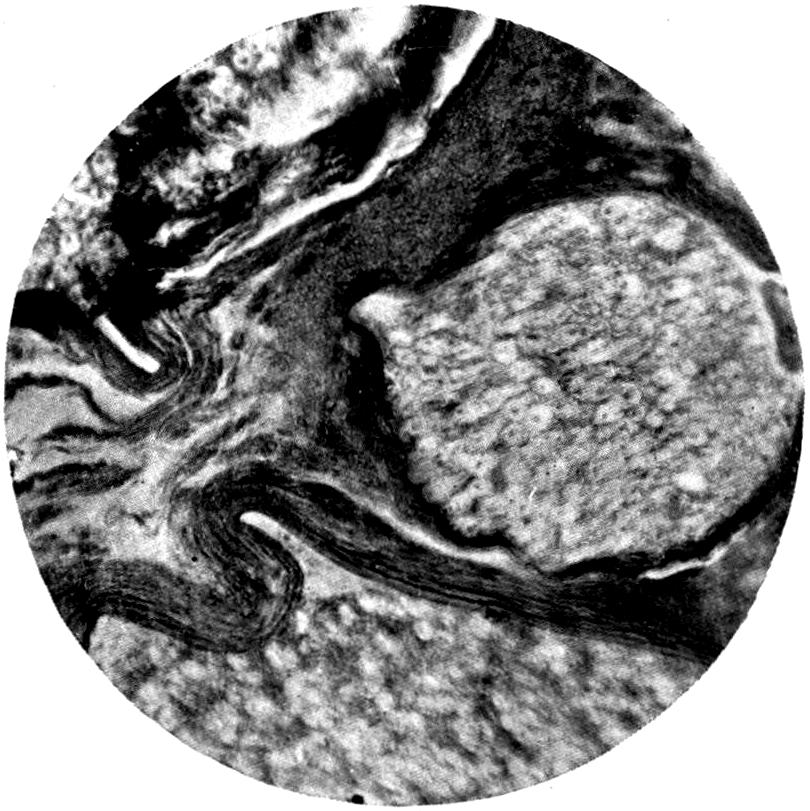
Fig. 3.—Section of Anterior Crural Nerve from Animal poisoned by Inhalation of White Lead Dust, showing Hæmorrhage in Nerve. (Stained Hæmatoxylin and Eosin.) × 250.
There was paralysis of the quadriceps extensor on the right side; the left leg was unaffected and the left anterior crural nerve was unaffected.
PLATE II
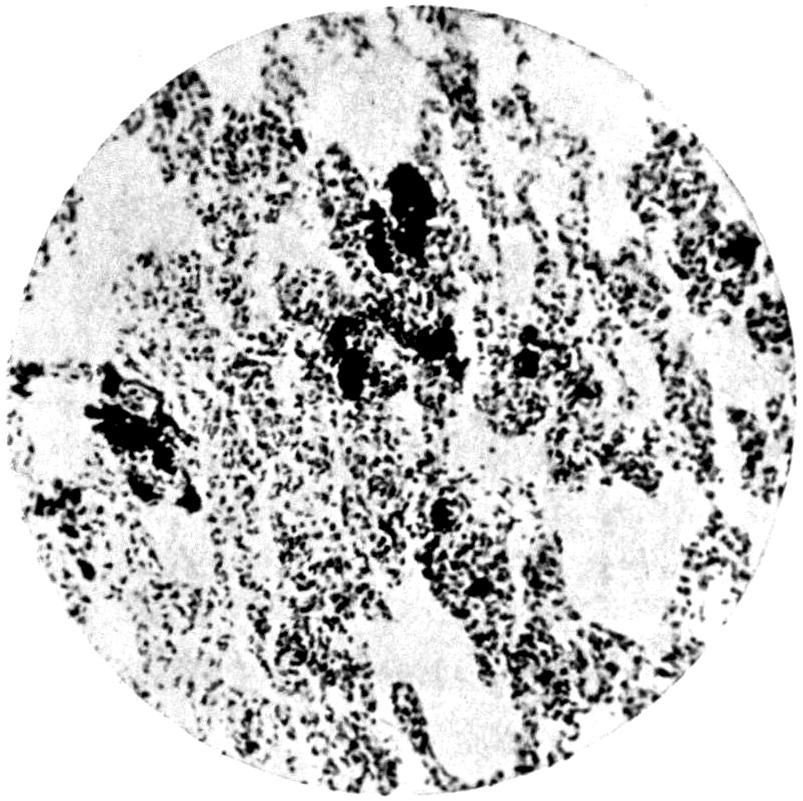
Fig. 1.—Section of Lung of Animal exposed to Inhalation of White Lead Dust, showing Mass of Lead in the Lung Substance. (Stained Hæmatoxylin and Eosin, and treated with H2S.) × 250.
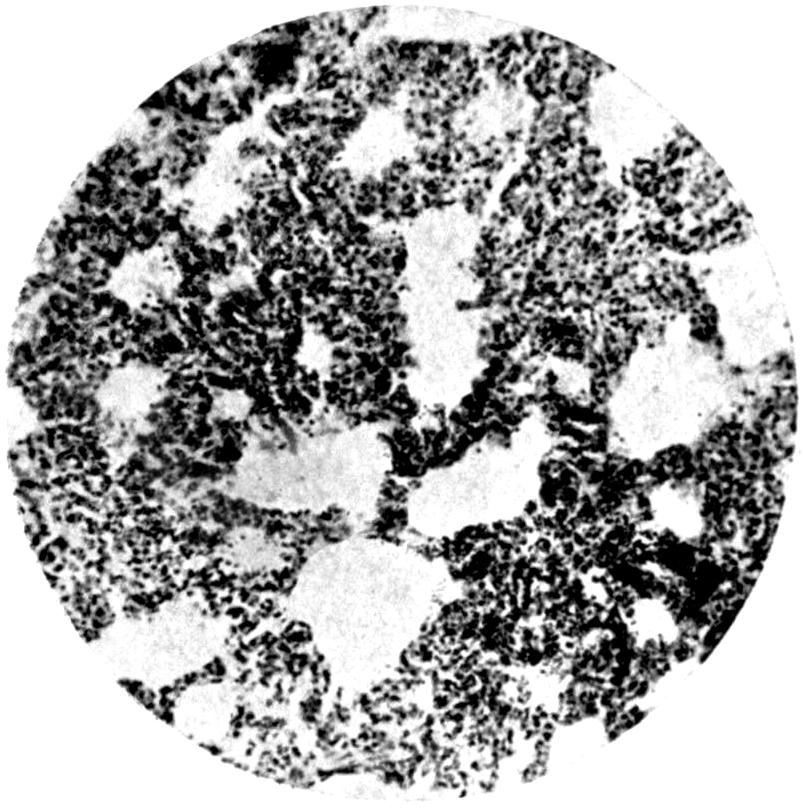
Fig. 2.—Lung of Animal exposed to Turpentine Vapour. (Stained Hæmatoxylin and Eosin.) × 250.
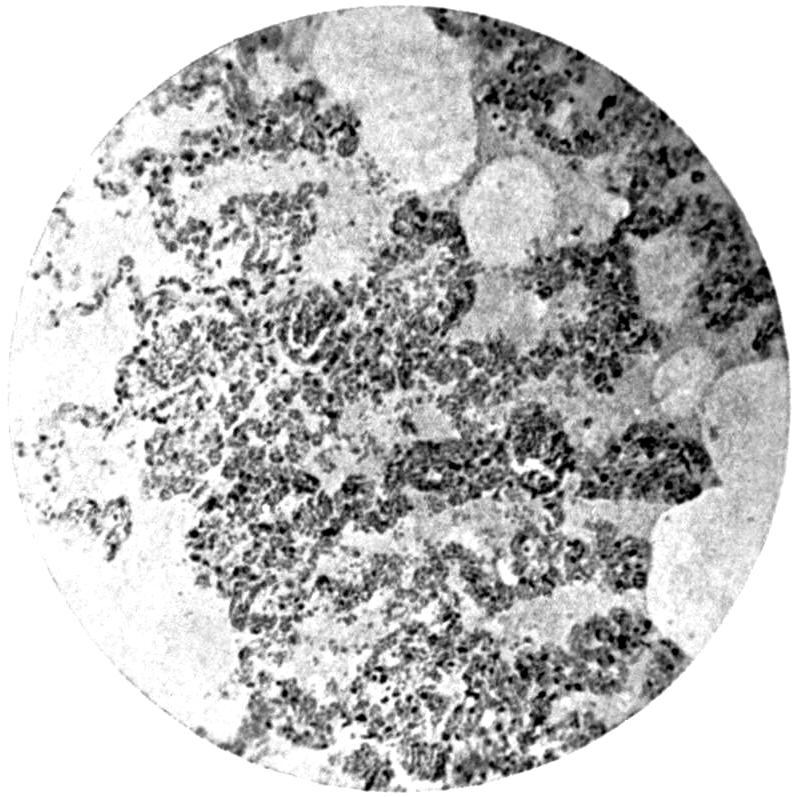
Fig. 3.—Lung of Animal exposed to Inhalation of White Lead Dust, showing Chronic Inflammation with Exudation and Capillary Leakage and Hæmorrhage. (Stained Hæmatoxylin and Eosin.) × 250.
[93]
Lung.—Red or grey hepatization may be found, or a general appearance of broncho-pneumonia, where the dust used contained angular or insoluble substances. In animals subjected to prolonged inhalation, particles of lead could be demonstrated in the alveolar cells, and in the tissue beyond, either by staining with chromic acid or by means of iodine. Staining by sulphuretted hydrogen is not very satisfactory, as most animals resident in a large city show masses of carbon situated in various parts of the alveolar cells. If, however, a section be treated by means of iodine or chromic acid, and watched under the microscope during the process, the particles of carbon are easily differentiated from those of lead compounds.
Nervous System.—Sections of the brain and spinal cord, and of the nerve supplying the paralyzed muscles, all exhibited the same phenomena of minute hæmorrhages. In later cases some change in the cells is found, but as a rule, beyond a slight increase of the intracellular substance, little or no change is found in the cellular elements of the brain; but in the region of the surface minute hæmorrhages may be constantly traced, spreading over the surface of the cortex. In the cord, sections made in various situations failed to show any very definite degeneration, and little or no hæmorrhage was observed amongst the cells of the cord. Hæmorrhages could occasionally be seen on the surface.
In a number of animals the anterior crural nerves supplying the paralyzed quadriceps extensor muscles were examined carefully both for degeneration and for hæmorrhages. Very few degenerated nerve fibres were found, not more than would be accounted for by the minute hæmorrhages, which were found passing in between the nerve bundles, and here and there producing pressure on the nerve bundles themselves.
Kidney.—In the kidneys minute microscopical hæmorrhages, some of them quite large, were found in the cortex. The hæmorrhages are diffuse and irregular, and apparently due here, as in other situations in the body, to the breaking down of minute venioles rather than arterioles. In many cases the change is capillary. Parenchymatous nephritis may be seen, probably resulting from the transudation taking place from the vessel walls.
The chief view brought out in the histological examination of the various organs is one of capillary hæmorrhage. This phenomenon is not peculiar to lead poisoning, but, from the work of Moore of Liverpool[4], it would seem that all[94] heavy metals, such as bismuth, mercury, not excepting iron, tend to produce a curious generalized yielding of the minuter vessel walls. Armit[5] has demonstrated a similar effect with nickel. The phenomena is, however, typically associated with lead poisoning, and may, we think, be regarded as the definite factor of chronic lead poisoning.
For the purposes of controlling the experiments of inhalation, two other series of experiments were undertaken. In one instance an animal was fed for two years on white lead; the animal was given 0·1 gramme per day, and this was increased up to 0·5 gramme, and ultimately 1 gramme. This animal exhibited no symptoms whatever of lead poisoning, and when it was killed, at the end of the time of experiment, showed no apparent lesion, with the exception of very marked staining of the colon and vermiform appendix. This staining of the large intestine and the appendix, the engorgement of the vessels, particularly of the omentum and mesentery, the enlargement of the lymphatic glands in the neighbourhood of the colon, ileo-cæcal valve, and appendix, suggest the absorption of lead in the upper part of the intestine, and its discharge or elimination into the large intestine. That lead is absorbed into the upper part of the intestine was demonstrated in the following manner:
An animal was anæsthetized, an incision made, and a loop of intestine pulled up and clamped off, a solution of lead chloride being run into the loop by means of a hypodermic syringe. The mesenteric vein passing from this loop of intestine was then carefully secured, a small opening made in it, and the blood collected drop by drop until some 40 c.c. had been collected, the time occupied being about three-quarters of an hour. The blood thus collected was submitted to chemical examination, and lead was demonstrated to be present. Lead therefore passes direct from the intestine into the portal circulation.
In only one of the feeding experiments with solid compounds of lead was any definite symptom of lead poisoning produced, and in this instance the compound used was dust collected from the flues of a blast-furnace. This dust was afterwards found to contain a considerable quantity of arsenic. The experiment cannot therefore be regarded as conclusive. With the more soluble salts of lead, however, such as the acetate, lead poisoning may be set up by means of lead administered via the intestinal canal: 1 gramme of lead acetate administered by means of a hypodermic syringe through a catheter passed into the stomach of a cat produced abortion in ten days, and death in three weeks. Four grammes of acetate produced a similar effect in a dog in four weeks.
[95]
PLATE III
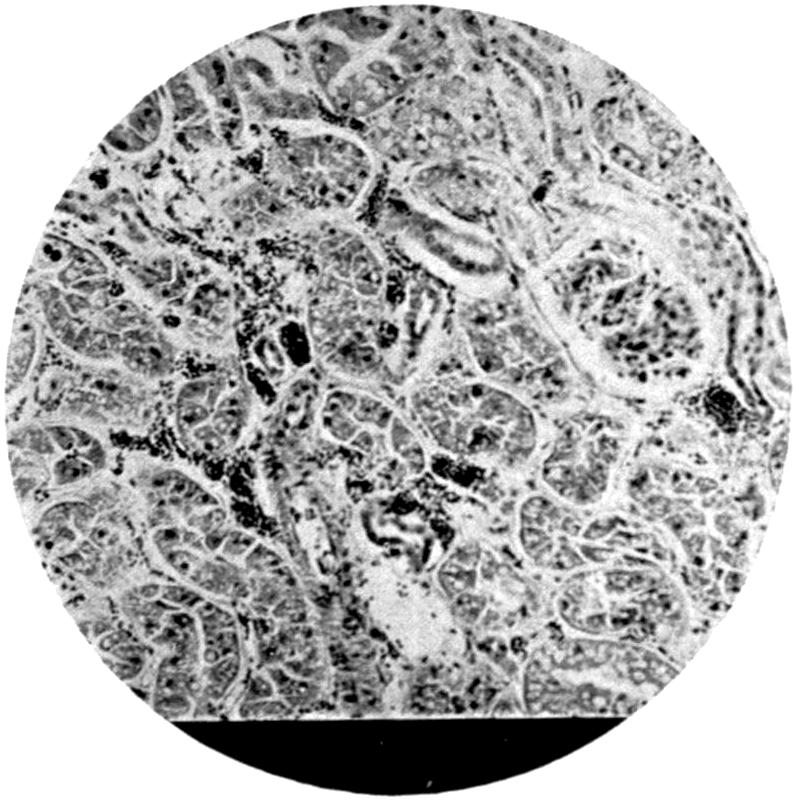
Fig. 1.—Kidney of Animal poisoned with White Lead (Inhalation), showing Microscopical Hæmorrhages. (Stained Hæmatoxylin and Eosin.) × 250.
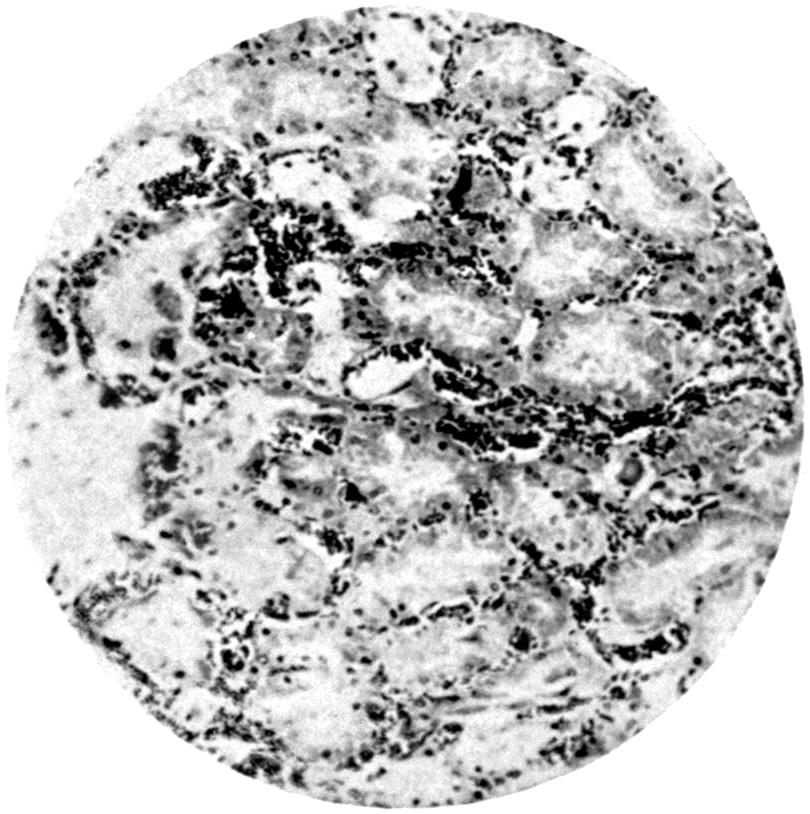
Fig. 2.—Kidney of Man dying of Chronic Lead Poisoning. (Stained Hæmatoxylin and Eosin.) × 250.
Fig. 3.—Brain of Young Woman dying of Acute Lead Encephalopathy, showing Small Cerebral Hæmorrhages. (Stained Hæmatoxylin and Eosin.) × 250.
The two following cases, in which both chemical and histological examination has been carried out on the tissues of persons who had been employed in occupations which rendered them exposed to absorption of lead, and who died with symptoms directly suggestive of lead poisoning, may be added, as they confirm the experimental results given above in all particulars:
Case 1.—A woman aged twenty-one, employed in a litho-transfer works, who died after a short illness during which the chief symptoms were headache and mental clouding.
At the post-mortem examination no pathological lesions were discoverable with the exception of a small gland which had become calcareous, situated near the right bronchus. The brain was injected over the left cerebral hemisphere, but no hæmorrhages were to be seen with the naked eye. There were no other pathological signs. A portion of the brain showing the injection and congestion were submitted to histological and chemical analysis. Histologically the brain tissue was found to be normal, with the exception of slight chromatolysis of some of the larger cells; but interspersed about the whole section in the slides examined, but more particularly in the area of the cortex, minute microscopical hæmorrhages were found (see Fig. 3). Here and there these hæmorrhages were seen related to the expanded capillaries, all of which showed considerable engorgement with blood. The arteries and veins themselves were, in addition, considerably distended. There was no interstitial degeneration of the neuroglia noticed. A few patches were found which apparently represented old hæmorrhages undergoing gradual fibroid degeneration. In no case were the hæmorrhages of a size that could be detected by the naked eye.
Two hundred and fifty grammes of the injected area of the brain were submitted to chemical examination by the moist process described in the chapter on Chemical Analysis. The nitric acid filtrate from the electrolytic deposition gave a well-marked precipitate with sulphuretted hydrogen, which was filtered off and recognized as lead. There was only the merest trace of iron present. By colorimetric estimation the quantity[96] of lead found present in the brain estimated as Pb was 0·0143 gramme. The quantity found in 250 grammes of brain substance examined from the injected area was 0·0041 gramme.
Case 2.—A man who had been employed in an electrical accumulator works for a considerable time, and who had had a history of several attacks of lead colic and one of lead paresis affecting both hands.
The man’s work in connection with lead ceased from the time of the paresis, but some three years subsequently he died with cerebral hæmorrhage.
Portions of the organs, brain, kidney, liver, and spleen, were examined histologically, stained in the ordinary way with hæmatoxylin and eosin. In the brain the same marked microscopical hæmorrhages were observed as described in the previous case, and in addition many more areas of old fibroid scars, very minute, but apparently corresponding to earlier minute hæmorrhages. The kidney showed definite interstitial hæmorrhage (see Plate III., Fig. 2), as did the liver and spleen.
A portion of the brain was further submitted to chemical examination, and 0·0014 gramme of lead was determined as present.
The importance of this confirmatory evidence is undoubted, as the presence of the capillary hæmorrhage existing in the tissues of a person dying under suspicious circumstances when employed in a lead process is confirmed by the chemical determination of lead in the tissues.
The following tables, arranged under three headings, give some of the experimental results obtained by submitting animals to the effect of compounds of lead.
Table XI. gives the inoculation experiments.
The materials used in these experiments were those used in the inhalation and feeding experiments. The experiments are also arranged in such a manner that each series is a control one of the other.
The amount of substance used for the inoculation gives some rough idea of the dose required to produce poisoning in an animal; but even this question of dose in absolute quantities, administered hypodermically, shows considerable variation in the degree of poisonous effect. The first animal in Table XI. was inoculated with acetate, this being one of the more soluble lead compounds, and was given it in three small doses. The animal[97] received 0·3 gramme per kilogramme of body weight, whereas in No. 35 2 grammes of washed frit—that is to say, lead glaze formed by fusing together litharge and silica—actually did produce symptoms, but of a mild nature. Animal No. 33 only had 0·16 gramme, being 0·05 gramme per kilogramme of body weight; and this caused acute symptoms. 0·35 gramme of white lead in a 3·500 kilogramme animal (No. 31) produced no symptoms at all. In the list of the inoculation experiments, three animals only exhibited no symptoms—one of these (No. 31) which was given white lead hypodermically, and Nos. 41 and 42, which were inoculated with lead silicate or lead frit, which had been previously treated with acetic acid or water.
Several practical points arise from these experiments, notably with regard to the frits, as it is seen that a considerable amount of the toxic properties contained in frit are removed by washing—most by washing with acetic acid and water, but also to some extent by washing with hot water alone, showing that in the ordinary production of lead frit for pottery purposes a certain amount of lead in a soluble condition as regards the body tissues was still present. This is no doubt entangled in the true silicates in the forms of oxides, or even as carbonate. Further, the toxicity of the lead compounds used may certainly be arranged in the order of their solubility with regard to the animal tissues, the acetate being the most poisonous, and the frit, when washed, the least; but unwashed frit, even in relatively small doses, may produce poisoning. This point is still further emphasized in Table XIII. Animal No. 42, four months after previous inoculation, showed no signs of poisoning. Lead nitrate in water was therefore given in quantities of 0·01 gramme per diem; one month later this animal developed poisoning.
Table XII. deals with the feeding experiments.
In these experiments acetate was given in one case only, and then in the form of pills coated with keratin. It is impossible to say, however, whether the animal ever received any soluble lead, as on one or two occasions the keratin pills passed right through the animal without dissolving. On the other hand, feeding with nitrate of lead in water produced symptoms, but when the nitrate was given in milk no symptoms appeared. It will be noticed it took a cat four months to show any signs of poisoning taking 0·1 gramme per day; whereas the animal receiving subcutaneous doses of 0·16 gramme of acetate[98] showed paralysis in fifteen days, and in twenty-two days was so ill that it had to be destroyed under an anæsthetic. The same relationship in time also obtains in the case of the animals fed on dry white lead. In practically no instance did definite or severe poisoning follow the feeding on dry white lead alone, even when quite large quantities were taken. On reference to the inoculation experiments of Table XI., it will be seen that the inoculation of 2 grammes of dry white lead produced definite symptoms, although the feeding cats had an amount very largely in excess of this. The only animals fed on white lead or frit exhibiting signs of lead poisoning were those which were given alcohol in addition to the lead compound.
A comparison of the results given in Tables XI. and XII. shows that animals which received lead compounds subcutaneously suffered much more than the animals which received the lead via the gastro-intestinal canal, even when the doses given via the mouth were exceedingly large. It will follow, then, that the actual contact with the more intimate fluids of the body, rather than the digestive juices, determines the solubility and general distribution of the lead compound in the body. This is confirmed by a recent paper by Straub[6].
The animals experimented upon by feeding were kept in the laboratory under the same conditions as the inhalation animals, but were so placed that under no circumstance could they obtain any lead dust by inhalation. These animals were used as control to the breathing experiments, the substance fed to the animals being in all cases the same substance as was used for the various inhalation experiments; but in addition a certain number of the animals were given alcohol, which are referred to in Table XII. Alcohol was also given to animal No. 6 in the inhalation series.
The animals fed with lead were fed with the same compound which was used for the inhalation experiments, 0·4 to 1 gramme being given daily; so that during the period these animals were exposed to lead dust the other animals were taking the same compound via the intestinal canal, but in much larger quantities, and yet they exhibited no signs of lead poisoning.
[99]
TABLE XI.—INOCULATION.
[100]
TABLE XII.—FEEDING EXPERIMENTS.
[A] Increased in weight.
[101]
TABLE XIII.—INHALATION EXPERIMENTS.
[102]
The experiments definitely bring out one all-important fact—namely, that lead dust circulating in the air is many times more dangerous than lead actually swallowed; for even if the animals which were exposed to the inhalation of dust swallowed the whole of the quantity contained in the respired air, they would only obtain one-tenth of the amount the other fed animals were getting. It is, of course, impossible to suppose that the whole of the lead contained in the inhaled air reached the lung. It can only have been the smaller particles which did so. Therefore the ratio is many more than ten times between the fed and the inhaling animal; in all probability only one-tenth of the contained lead in the respired air reaches the lung. Under these circumstances the ratio of poisoning via the lung to poisoning via the intestinal canal is as 100 : 1.
Table XIII. deals with the question of inhalation.
Every care was taken during these experiments to avoid any vitiation of such experiments by the actual swallowing of lead dust by the animals exposed to breathing. Moreover, all the animals were carefully controlled, in that an animal of somewhat similar weight at the same time was subjected to the ingestion of the same lead compound, but in much bigger quantities, via the mouth.
It will be seen immediately, on comparing Tables XI. and XII. with Table XIII., that the rate of poisoning by means of dust is greatly in excess of the rate of poisoning by feeding, even where poisoning by feeding actually occurred. Also that the amount of dust present in the air inhaled shows a marked correlation with the date of appearance of the first symptoms of poisoning, and that where the quantity of dust is very much reduced the poisoning was delayed longer than might have been expected, and that when poisoning did appear the symptoms were much less pronounced than with the more dusty atmospheres; and this although the quantity of lead obtained would be relatively the same over the range of time the animals were exposed.
The knowledge gained in dealing with industrial poisoning clinically receives very strong corroboration from these inhalation experiments, for it is a well-known fact that many persons engaged in dusty trades exhibit a species of immunity to lead poisoning. It is true that some susceptible persons, as has already been pointed out, very rapidly show signs of poisoning, even with a dosage producing no effect in other persons working under similar conditions; and it is highly probable that these persons have arrived at a species of equilibrium by which they are able to excrete the lead ingested, and so prevent any accumulation and general damage to their tissues. Directly, however, the dosage is increased, signs of poisoning come on, as in the[103] case of animals Nos. 10 and 11. For some seventy to eighty days little or no sign of poisoning was seen with the small dosage commenced with. At the end of this time, as no symptoms appeared, the quantity of lead in the air was increased, with the result that poisoning rapidly became manifest.
We have also in these inhalation experiments, in Cases 21 and 22, definite evidence that a low-solubility glaze—that is to say, glaze containing fritted lead—is capable of setting up lead poisoning when taken via the lung, as when such glaze is inoculated, although it produces no such symptoms when given via the mouth, except, perhaps, when it is complicated by excessive alcohol.
—The cat is peculiarly susceptible to lead poisoning. In lead works it is impossible to keep a cat any length of time, as it rapidly dies of poisoning.
All the animals subjected to lead absorption, and definitely suffering from symptoms of lead poisoning, exhibited the following symptoms:
1. Slight increase of weight over the first period of poisoning, lasting from one to three weeks.
2. A progressive diminution in weight, progressing until the animal exhibited definite signs of poisoning.
3. Wasting, especially of the spinal muscles (the erector spinæ and in the lumbar region), out of proportion to the determined loss of weight; pinched facies, with frequent exhibition of running from the eyes and nose, even when not exposed to the action of lead dust, merely by inoculation.
4. Various types of paralysis, particularly in the cat; the muscles of the back and of the quadriceps extensor of the hind-legs show signs of paralysis. In the cat the quadriceps extensor is paralyzed sooner than the extensor communis digitorum in man. The cats show loss of power in the hind-limbs by inability to jump. The reflexes, particularly the knee-jerks and elbow-jerks, are first of all increased, and latterly become lost.
The chief and main sign which was noted in the histological examination of the animals inoculated was one of minute microscopical hæmorrhages (this has already been referred to); these hæmorrhages were not confined to any particular position in the body nor to any one organ. In the animals which showed symptoms of epilepsy, occasionally thickening of the pia mater[104] was found, but invariably in such cases small hæmorrhages were found immediately under the arachnoid, not covering any great area, but apparently causing pressure upon small areas of the cortex. In others, again, the hæmorrhages were found lower down in the brain, and a few in the spinal cord. At times a large amount of hæmorrhage was to be found present at the base of the brain, spreading downwards from the medulla into the spinal canal, but this only occurred in such animals as died with encephalopathic symptoms. In animals which had signs of more chronic poisoning—that is to say, gradual loss of body weight, emaciation, constipation, contraction of the abdomen, and paresis, particularly of the hind-limbs and the muscles of the back—hæmorrhages were found in the muscles, liver, spleen, lung, heart, various positions in the abdomen, in the spinal cord, in the nerve-supply of the affected muscle, and even in the brain, none of them large enough to produce absolute destruction of more than a very minute portion of the organ in which they were situated.
Now, all these symptoms, and, more important still, the phenomena of hæmorrhage, were found in all the animals which exhibited similar symptoms, whether they were poisoned by inhalation of dusty lead compounds or fed upon lead compounds associated with alcohol; but even in some of the animals which were fed upon lead compounds—particularly white lead—and which had exhibited no definite symptoms of paralysis, or, for that matter, any symptom referable to poisoning, here and there slight histological changes which were referable to minute hæmorrhages.
The experimental work therefore carries us very considerably forward in correlating the symptomatology and pathology of lead poisoning. The symptoms produced in susceptible animals by the actual inoculation of a lead compound differ only in degree and rapidity of onset from those produced in animals submitted to inhalation with similar compounds. Feeding, on the other hand—that is, ingestion by way of the gastro-intestinal canal—even in large quantities, did not produce poisoning to any great extent, except when some material such as alcohol was added, thereby breaking down the animal’s resistance. Another interesting fact is given—that if lead is taken by the mouth in addition to milk a great deal of the poisonous effect is got rid of; thus of two animals—Nos. 46 and 47—which received[105] lead nitrate in their food, the one in water and the other in milk, the one which received it in milk showed no effects even after four months’ experiment, whereas at the end of four months the animal which was receiving the compound in its water died. This brings out a point already insisted upon—namely, that in all lead factories it is highly important that no work should be undertaken first thing in the morning, before the workers have had a proper meal, and that in the absence of a proper meal milk is the best substitute. It is highly probable that the soluble lead salt becomes united in some form of albuminate which is dealt with later, and perhaps turned into a sulphide and excreted without absorption. There is no possible doubt, from the large series of experiments which I have performed, that lead inhaled is far more poisonous than when absorbed in any other way; further, that the amount of poisoning produced differs somewhat according to the type of compound inhaled, and the experiments, moreover, give some suggestion as to the dose which is likely to produce poisoning. It is seen, where the animal is inoculated with white lead, the dose required to produce symptoms is below 1 gramme per kilogramme of body weight, but above 0·2 gramme per kilogramme of body weight. In feeding, 0·8 gramme, and even 1 gramme, per diem for eighteen months produces no effect, although the same quantity plus an excess of alcohol rapidly produces the disease. On the other hand, as small a dose as 0·1 gramme of nitrate of lead given in water for four months produced death.
Turning to the inhalation experiments, the quantity of dust breathed when as high as 0·0007 gramme per litre produced symptoms after only twelve inhalations for a period of about thirty-seven days; whereas when the dose was reduced to 0·0001 gramme per diem the time required to produce symptoms of poisoning was 120 days; in fact, this last dose (0·0001) for the animal under experiment was almost the lower limit, as this animal showed an almost steady line of weight for a considerable time, the weight remaining up for the first hundred days, a slight variation taking place from week to week until a progressive diminution set in.
Practically all the animals poisoned manifested a very distinct diminution in body weight; in four only other symptoms of poisoning appeared first. This is a fact that is often to be noted amongst lead-workers, and if a progressive diminution in weight[106] takes place, there is strong reason for supposing that a considerable alteration in the metabolism of the body has taken place; but it does not follow that microscopical hæmorrhages or other definite effects of poisoning are present, although such is probable.
Finally, in summing up the conclusions to be drawn from the above experiments, it has been suggested that such experiments as inoculation, experimental inhalation, or even feeding, are no criterion of the circumstances under which industrial workers become infected with lead. It is perhaps hardly necessary to refer to this point, but for the fact that it is possible this book may be made use of by those who are not in the habit of dealing with experimental pathology. One of the first and most important matters in dealing with any form of poisoning is to obtain knowledge of the actual symptoms both clinically and physiologically, as well as pathologically, of the effects of any drug, and to determine if the symptoms so produced in an experimental animal conform to the symptoms as seen in man. For the purpose, therefore, an animal is required which is susceptible to the poison, and therefore cats were used in the foregoing experiments, as it is absolutely impossible to keep a domesticated cat in any white lead works, for the animals invariably become poisoned by lead.
The second point in prosecuting an inquiry into the pathology of any disease is to determine the train of poisoning when definite dosage, both in quantity and compound, is made use of. By feeding an animal with a compound only, the absorption through the gastro-intestinal canal could be studied; whereas by inoculating some of the compound—in suspension if it be insoluble, or in solution if it be soluble—into the subcutaneous or muscular tissue, the direct action of the body fluids on the compound may be studied; and, furthermore, its absorption by the membranes—that is, the cell membranes and the animal tissues—are determined. It is necessary to give at first a dose big enough to produce definite symptoms, and then to gradually decrease the dose to find the minimum amount producing symptoms within a reasonable amount of time. Inoculation experiments therefore give an answer to a number of these questions, and are the basis upon which further inquiry is conducted; they form a criterion from which it is possible to judge the effect of inhalation, and the same remarks which have been made with regard to inoculation refer to inhalation experiments. It is essential first[107] of all, in the experimental animals, to subject them to rigorous enough conditions to obtain definite symptoms, and then, by varying the experiments, to study the amount, entrance, and general behaviour, of the poison, correlating the evidence so obtained from the definite knowledge already gained with the previous experiments.
It is hoped that this brief note on experimental evidence will assist in the elucidation of the foregoing experiments to those who are not conversant with the application of experimental evidence.
—A series of further experiments were made, with particular reference to lead poisoning exhibited by painters; and as these experiments and their results could not have been undertaken without the previous knowledge gained of the pathology of lead poisoning due to the inhalation of particles of dust floating in the air, their discussion has been reserved until the previous section had been dealt with.
It has been supposed by some that surfaces painted with lead paint give off certain emanations containing the metal lead as an organic compound. As the incidence of lead poisoning amongst painters is exceedingly high, as far as any statistical evidence can be obtained (see p. 48), it would seem that the painter is peculiarly exposed to infection by lead dust; and if, in addition, organic compounds of lead were given off, he would be still more liable to lead poisoning.
Two methods of experiment were used:
1. The exposure of animals to the fumes given off from freshly painted surfaces, the paints used being compounded with white lead, lead sulphate, zinc sulphide, and zinc oxide.
Animals were exposed in a cage similar to that used in the inhalation experiments previously described, but, instead of blowing in the contaminated air, the cages were so arranged that boards freshly painted with the special paint under experiment were introduced into the cage daily, the animals remaining the whole time in the chamber. Special precautions were taken with regard to ventilation.
2. An animal was placed in a chamber, and the compound to be tested was heated electrically by means of a coil surrounding the glass tube in which the compound was placed. The current was regulated by means of resistances, so that the thermo-couple and galvanometer gave a constant reading of 59° C. Air was constantly[108] passed through the tube over the heated substance and into the animal’s cage, which was efficiently ventilated. In this way any emanations which were given off from the normal room temperature or up to 59° C. were carried over into the animal’s cage, and there breathed. The apparatus was so arranged that the heating coil extended close to the point of delivery into the cage.
The result of these experiments showed that the animals confined in cages and exposed to freshly painted surfaces, where the paint used was white lead, zinc oxide, or lead sulphate, very soon showed signs of poisoning, and they became emaciated and suffered from recurrent attacks of salivation. The animals exposed in the cages in which air was passed over either white lead paste, zinc paste, or lead sulphate paste, showed no signs of illness, although kept in the cage and subjected to the inhalation of any fumes which might be given off for three months, spending the whole of the day in the cage, but being removed during the night to separate cages.
It therefore seemed clear that, whatever illness was produced in the animals exposed to fresh paint, they were not suffering from absorption of lead, but of some other compound of which the paint was made. Various constituents of the paint were therefore tried—namely, the metallic bases, lead or zinc, and linseed-oil, with turpentine and lead acetate mixed with turpentine. The animal exposed to the turpentine alone very rapidly showed signs of disease—salivation, a tendency to diarrhœa, strabismus, but the latter only after a two-hour exposure, whilst the quantity of turpentine present in the cage air did not exceed 10 milligrammes per litre.
The animal exposed to turpentine and lead acetate exhibited few symptoms, but the same in kind as the animal exposed to turpentine alone. The linseed-oil animal showed no signs of disease whatever. The animals exposed to the metallic bases of the paint—namely, zinc oxide or white lead—showed no signs of poisoning as long as the compound itself was not thrown into the air in the form of dust; but when lead dust was present in the air the animal rapidly showed the ordinary signs of lead poisoning. The animal exposed to zinc oxide dust showed very little sign of discomfort, but by prolonged exposure early kidney disease was produced, and signs of chronic inflammation were detectable in the lung.
It is interesting to note in this connection that Lehmann[7] describes symptoms produced in cats when exposed to the vapour[109] of turpentine. The animals which I exposed to turpentine vapour exhibited the same symptoms as those described by Lehmann. He gave no result of the histological inquiry of the animals so exposed, but in no case, apparently, was the animal killed after exposure. In my animals exposed to the vapour of turpentine very definite disease of the kidney was produced, the inflammation tending rather to the tubular than the interstitial variety of nephritis. The tubules were found blocked with débris, their contour irregular and destroyed, and their substance pale and almost hyaline; whilst areas of cloudy swelling, together with small hæmorrhages, were to be found scattered about the kidney. The heart muscle was flabby, and the heart tending to dilatation; whilst microscopically hæmorrhages could be found throughout the organ of a minute capillary nature, and passing between and disturbing the muscle bundles.
No changes of any sort were found in the tissues of the animals exposed to the emanations given off from white lead paste. By analyses these emanations were found to contain no lead, but traces of aldehyde, formic acid, and CO2. It follows, therefore, that the effect of turpentine when inhaled by the painter must be to act as a contributory cause of lead poisoning, and it is interesting in this connection to recall the fact noted on p. 38, that Garrod has described gout as occurring constantly among painters. The statement already quoted, that gout is not common among workers in white lead factories, where the exposure to lead is very much greater than among painters, points to turpentine as the cause of the increased incidence of gout among house-painters rather than lead absorption. The importance of dust containing lead as a source of illness and lead poisoning in painters must not be minimized, as in sand-papering, etc. (see p. 137). The importance of lead dust inhaled in this way is perfectly understood. It is, however, highly probable that the combined action of the turpentine with the lead accounts for the fact that headache is a common symptom of early disease in painters, which is not the case among white-lead workers.
[1] Goadby, K. W.: Journal of Hygiene, vol. ix., No. 1, 1909.
[2] Goadby, K. W., and Goodbody: The Lancet, vol. ii., p. 988, 1909.
[3] Goadby, K. W.: Report of the Committee on Lead, etc., in Potteries, vol. iii., p. 478, 1910.
[4] Moore: Private communication.
[5] Armit: Journal of Hygiene, vol. viii., No. 5, 1908.
[6] Straub: Berl. Med. Woch., p. 1469, 1911.
[7] Lehmann: Archiv für Hygiene, vol. xxxiv., p. 321, 1899.
[110]
—Acute poisoning by lead is not common. Industrially it hardly ever occurs. Zinn[1] states that, out of 200 cases of industrial lead poisoning in his clinic in Berlin, only one was to be regarded as an acute case. In most instances the poisoning is due to swallowing some compound of lead, either as an abortifacient or to commit suicide.
The pathology and symptoms of such acute lead poisoning depend in the first place upon the nature of the salt of lead swallowed, as, for instance, after swallowing sugar of lead a burning taste is complained of, with acute gastric pain, generally coming on within an hour of taking the poison, salivation, metallic taste in the mouth, acute hiccough, and griping pain in the abdomen. The mouth is stained a whitish-grey. Later there is a great fall in blood-pressure, the skin becomes moist, or a cold sweat may appear. The respiration and pulse drop; finally comes vertigo, acute headache, coldness of the extremities, anæsthesia, and death in one or two days, or the case passes on to one of chronic poisoning. If the patient survives for the first two or three days, retinal changes frequently make their appearance, and occasionally acute fever may supervene. Various paralyses also appear, and the case then becomes one of subacute or chronic poisoning.
The lethal dose for healthy adults is probably as large as 50 grammes for lead acetate, for lead carbonate 25 grammes; these doses are, of course, only approximate.
Post mortem in a case of acute lead poisoning by ingestion, the mouth and stomach show the presence of a lead salt in the mucous exudation, together with corrosive gastritis and considerable swelling and œdema of the mucous membrane. The large intestine is generally stained darkly, from a light brown[111] to a deepish black; this staining may not appear until quite low down in the intestine. There is hyperæmia of the liver, much engorgement of the vessels in the mesentery, the kidney, and the brain. The rest of the intestinal viscera show signs of engorgement. Fluid may be present in the peritoneal cavity, and occasionally in the other serous cavities.
The histological examination of the various organs exhibits the same microscopical hæmorrhages as are found in the cases of chronic poisoning to be described later.
Although acute lead poisoning is rare in industrial experience, it may occur from time to time. Several cases are on record where a workman developed an acute attack of poisoning as a result of immersion in a white lead beck; another case is described which followed immersion in a tank of solution of lead acetate.
An accident of this description may conceivably occur; the treatment of such a case should be energetic, as the poisoning is chiefly due to lead swallowed. An emetic should be given, followed by sublimed sulphur, or, better still, the stomach washed out with dilute hydrogen sulphide water slightly acidulated with sulphuric acid, so as to change any lead present in the stomach into the least soluble form. A brisk purge should be given, and the patient encouraged to drink considerable quantities of lemonade containing sodium or potassium citrate. Alcohol, even during collapse, should be avoided; a hypodermic injection of strychnine is preferable. It must be borne in mind that lead is absorbed in the upper part of the intestine, and only in a minute degree, if at all, from the stomach; it is re-excreted mainly by the large bowel and by the urine; to some extent also by the sweat and saliva. Treatment is therefore directed towards (a) forming an insoluble compound as far as possible; (b) promoting the elimination of the poison; (c) placing as little work as possible upon the tissues most affected. Only milk should be given as food for two to three days.
The diagnosis of lead poisoning is not in itself of any great difficulty where any one of the classical symptoms of lead poisoning is present, such as lead colic, paresis, or the characteristic lead anæmia or cachexia. On the other hand, the premonitory symptoms of poisoning, as seen by a club doctor, particularly in persons engaged in industrial processes, are more difficult to determine; but for the appointed surgeon, who has an opportunity[112] of watching them from week to week, the gradual development of anæmia, extensor weakness, and other early symptoms, should give no difficulty. The clinical diagnosis requires to be made earlier by the appointed surgeon than by the general practitioner; for the appointed surgeon’s duty in a white lead works is not only to treat lead poisoning when it is once established, but, by carefully noting premonitory signs, to avoid the development of actual symptoms in susceptible persons. It is convenient, therefore, to divide the diagnosis of lead poisoning, from the clinical standpoint, into two divisions—incipient and pronounced. Such incipient changes are for the most part noticed amongst lead-workers, and in many cases are more strictly signs of lead absorption than signs of lead poisoning.
The earliest symptoms of poisoning are found in the vascular system, and the curious pallor of the face in persons who have worked in lead for a considerable period is often pronounced, although the conjunctiva may not show such a diminution in colour as might be expected from the facial change, while the actual determination of hæmoglobin may be almost normal. In addition to this, a person of fresh colour working in lead, if susceptible, very quickly loses his florid appearance, often heightened by the colour of the face only remaining on the cheek-bones as a hectic flush. Such a person will also show diminution in the colour of the conjunctival vessels, and invariably a distinct yellowish appearance of his sclerotics, due to the pigmentation of that tissue by broken-down blood-pigment. The yellow colour of the eyes is definite evidence that blood-destruction is in progress.
Following on the anæmia, or, more strictly speaking, the pallor of the face, a well-marked wasting of the subcutaneous fat takes place. In animals poisoned with lead in small or large doses—particularly in small doses given over a considerable period—this wasting of all subcutaneous and other fat is a very marked feature, so much so that practically no kidney, mesenteric or abdominal fat is to be found. The fat is lost in a greater proportion than other body tissues. In man the infra-orbital fat, together with the fat about the buccinator muscle, suffers early, and a curious facial contour is produced, two well-marked folds being seen—one the ordinary naso-labial fold, and the other situated at the anterior margin of the masseter. This, together with the loss of the orbital fat, gives the face a curious pinched appearance.[113] Such a pinched appearance is also to be found in animals (cats) poisoned by lead. The wasting frequently precedes any other symptoms, and it is no uncommon thing to find that a man who has been working in a lead process exposing him to inhalation of dust for a year is losing weight. In one case a man of 10 stone 7 pounds was reduced in weight to 9 stone 2 pounds in fourteen months, during the whole of which time he showed no signs at all of lead poisoning, and only towards the latter end of the time did he exhibit any blue line on his gums; there were no symptoms referable to lead poisoning. Such a case is typically one of lead absorption, which, if continued, would ultimately result in pronounced anæmia with either colic or paralysis, probably the former. The man in question was not engaged in any lead process, but was an electrician attending to the electric light and motors in a smelting works. His occupation necessitated work above the general ground-level, and therefore he inhaled the fumes and finer dust particles detached especially from the arc lamps which required adjustment.
In many persons who have worked in lead for long periods, wasting does not progress beyond a certain point, and these persons may be regarded as having established a certain degree of immunity. Men are met with who have worked in white lead factories and in smelting works for periods of from twenty to, in one case, forty-three years, a considerable portion of such period being antecedent to the time at which the regulations in force for dust removal and general protection of the workers were established, and they must have been exposed to much lead dust. Nevertheless they were less emaciated than many who have only worked a year or two years in a factory under modern hygienic conditions and special regulations. Such persons are either immune from the commencement, or they have established a certain degree of tolerance towards the metal; the latter supposition is the more probable, as there is reason to think several of them suffered from a mild degree of poisoning during the earlier years of their employment.
The rate of development of pallor and wasting are the important facts in incipient poisoning. Anæmia, together with the presence of basophile granules in the red cells, in a previously healthy person, and diminution in the hæmoglobin to 75 per cent., is definite evidence that absorption is leading on to poisoning—that is, blood-destruction—and coincidently insidious damage to[114] the finer bloodvessels and their nerve-supply (see Chapter V.). Such a person may at any moment develop a sudden attack of colic or paresis.
Associated with the wasting and pallor comes wasting of the muscles themselves, quite apart from any nerve lesions, and with it a certain degree of mental lethargy, slowness in comprehension, and loss of power over individual muscles or groups of muscles, more usually the latter. The mental lethargy shows itself in many ways—amongst others, heaviness and drowsiness—and careful examination should be made of any man who, previously a good time-keeper, commences somewhat suddenly to be late in the morning. The muscles of the hands and the arms, may show no definite wasting as compared one with another; but early loss of power, particularly of the extensors of the wrist or fingers, may be present for six months to a year before definite wrist-drop makes its appearance. In two cases under our own observation, loss of power of the extensor muscles of the wrist was present—in the one case for eight, and in the other case eleven, months before definite paresis occurred. In the one case the first symptom of paralysis was inability to extend the little fingers of both hands; the case was at once suspended from work, and was given treatment. Within forty-eight hours both wrists were so far affected that they could not be extended. In the second case the first symptom of paresis other than the loss of extensor power was the inability to oppose the thumb to the first index-finger of the left hand; within seven days complete wrist-drop of the left hand, partial drop of the right hand, including the middle and ring fingers, occurred. Both cases made a complete recovery.
By no means all wrists which show weakness of the extensor muscles ultimately develop paralysis; for, on looking through the records of the examination of three factories, only 4 per cent. of persons whose wrists were noted as showing loss of extensor power ultimately developed definite paralysis. In the extended position of the hands, when the surgeon is examining for extensor weakness, note should be made of any tremor, as fine tremor is frequently an early symptom of ensuing paralysis. The tremor is generally fine, increased on attempting to perform concerted acts (intention tremor); some loss of co-ordination may be found.
Where the nerve supplying the muscle has suffered alteration in its conductivity, as by the occurrence of a small hæmorrhage in[115] its sheath, gradual diminution in the nutrition of the muscle takes place; but whether or not this is sufficient explanation for the chronic wasting which is seen in the hands is not yet definitely proved.
In examining the hands outstretched for tremor and loss of muscular power, wasting of the interossei may be seen before any definite evidence of paralysis supervenes. On the palm of the hand both the thenar and the hypothenar eminences may show flattening, and attention should always be paid to this portion of the hand, as an early flattening of the hypothenar eminence particularly is one of the earlier symptoms of wrist paralysis.
—Constipation is a well-known precursor of colic in lead poisoning cases, but is by no means an invariable rule. About 15 per cent. of cases of lead colic suffer from intermittent diarrhœa.
A number of cases (see the table on p. 49) show that “rheumatic” symptoms are amongst those associated with lead poisoning. Some of these rheumatic symptoms, as has been explained, may be due to minute hæmorrhages, in the muscle or elsewhere, setting up the pains of a rheumatic nature in the part affected. One other symptom occurs with considerable frequency both as a precursor of colic and as an associated symptom in constipation, and even in diarrhœa associated with lead poisoning, and often regarded as of rheumatic origin—namely, lumbago. Complaints of lumbago in lead-workers should always be regarded seriously, as they may be a guide in discovering an early intoxication.
From what has been said of the excretion of lead into the large intestine in poisoned animals, the symptom of lumbago often complained of by lead-workers may, in some instances at any rate, owe its origin to overloading of the large intestine, due to the inhibitory action of lead on the intestinal muscles. It has been seen that excretion of lead into the large intestine is the normal method of excretion of the metal, and that concomitant congestion of the vessels in the corresponding mesenteric area is an associated symptom in poisoned animals. Local vasomotor spasm may also contribute.
—The pulse in lead poisoning in the incipient stage is perhaps not so important as when poisoning has become pronounced. Considerable variation exists amongst different observers with regard to the blood-pressure of lead-workers. Our experience is, on the whole, that it tends to be high,[116] and pressures of 150 to 170 mm. Hg are common. In an average of 100 cases we found the highest pressure to be 178, and the lowest 115, the mean 150.
Collis[2], in a special report on smelting of materials containing lead, gives the average blood-pressure of 141 smelters as 148·2, and of 38 white lead-workers as 156·5.
Increase of tension undoubtedly takes place as lead absorption becomes more established, and the well-known high arterial tension of arterio-sclerosis is to be found in most workers employed in lead for any considerable period. Even in the cases quoted above showing no signs of lead poisoning, notwithstanding long duration of employment in lead factories, there was a distinct increase of arterial tension, not necessarily attributable to lead alone, but possibly also to such incidental causes as gout, alcoholism, syphilis, etc. When colic is present, marked diminution in the pulse-rate may be noticed during the spasms, and even without the presence of colic a diminution in the pulse-rate, with a definite increase of tension, as estimated by the finger, is a matter of practical importance in the diagnosis of absorption.
In quite early stages the pulse may be increased, and a small, rapid pulse should be regarded as a suspicious sign. Only in the later stages of the disease do alterations in the heart-sounds take place.
Sphygmograph tracings of the pulse of lead-poisoned persons shows the well-marked high tension type.
—Probably the commonest symptom, and the one for which first and foremost relief is sought, is abdominal colic. The colic of lead poisoning, when once seen, is rarely mistaken a second time. The pain is generally referred to the lower portion of the abdomen, low down, and often the sufferer points to a position immediately above the pubes; pain may often be referred also to the right iliac fossa, and on this account the possibility of appendicitis, or even perityphlitis or chronic colitis, must not be forgotten.
Colic, as shown in the statistics on p. 49, is the chief symptom complained of. No doubt, as has been previously suggested, the fact that colic is so common a symptom induced earlier pathologists to regard the entrance of lead through the gastro-intestinal canal as being the portal of lead infection. We have, however, demonstrated in the chapter on Pathology that the absorption[117] of lead, particularly in industrial processes, is mainly by way of the lung, and in the résumé of the literature it has already been pointed out that strong evidence exists for regarding colic and other abdominal symptoms associated with pain as due to vaso-motor disturbances in the splanchnic and mesenteric areas.
Lead colic is usually irregular, with marked exacerbations and remissions, and in the acute form the legs are drawn upwards towards the abdomen, the body is flexed at the hips, the face anxious and drawn, whilst the body is covered with a cold sweat, the eyes are staring, and convulsive movements of the limbs occur. The sufferer often finds relief from firm pressure upon the abdomen, a point of considerable importance in diagnosis; the pain is not increased, but distinctly relieved, by firm pressure.
If the abdomen be digitally examined during a paroxysm of pain, the intestines will be found contracted under the fingers, often in an irregular fashion. In an animal acutely poisoned with lead the intestines are found irregularly contracted during a very large portion of their length, and when removed from the body have the appearance of a string of sausages. There is evidence that spasmodic contraction of the circular fibres of the muscles of the mucosa has taken place, and the wave of peristalsis, moving forward, meets with a block at these points of constriction, thereby causing pain.
The colic affecting the lower part of the abdomen may possibly be related to the excretion of lead into the large intestine, and the affection of the bloodvessels in the mesentery, and in animals poisoned by means of lead the vessels in the mesentery, particularly in the region of the ileo-cæcal valve and the large intestine, are engorged with blood.
During a paroxysm the patient frequently screams in agony and rolls about upon the bed or the floor. Temporary relief may be obtained by leaning upon a pillow placed upon the back of a chair. The relief afforded by such procedure is also strongly in favour of the vaso-motor origin of the pain. During the spasm the abdomen is retracted, and fibrillary twitching of the abdominal parietes may often be seen; a constant desire to go to stool is generally present, but only results in straining and, perhaps, the passage of a little mucus and blood.
Vomiting is often associated with this stage, and the patient frequently vomits a considerable amount of thick, tenacious mucus. The vomit is not uncommonly regarded by the patient[118] as composed of white lead, if he has been working in that industry. Tanquerel in 1,217 cases noted vomiting 400 times, and marked retraction of the abdomen 649 times. Occasionally the patient complains of a sense of great weight in the abdomen, particularly in the intervals between the spasms of pain.
During the exacerbations of colic very marked diminution in the pulse-rate takes place, a fact that has already been referred to in the section dealing with vaso-motor disturbances. The pulse may be as low as 20 beats per minute, but it varies generally between 40 and 50 per minute.
Very occasionally the first stage of colic is associated with a slight rise of temperature. This must be regarded as an intercurrent affection rather than as one definitely associated with lead poisoning. Probably in such circumstances a gastritis other than the lead vaso-motor colic is the reason for the elevation of temperature, but it may confuse the diagnosis, suggesting rather an acute gastritis than lead colic. Under normal conditions the temperature falls during the colic, the extremities are cold, and the body is covered with a moist perspiration, the temperature dropping to 96°, and even lower.
On palpating the abdomen during these acute exacerbations, it is found that not only the gastric region, but the whole of the abdomen, is affected. Occasionally the acute pain is referred to the navel, but generally to the lower region of the abdomen; and very frequently the pain is described as running down into the scrotum, whilst there may also be pain complained of as far as the knee-joint, but this last is unusual. There may or may not be well-marked peristalsis taking place, but quite commonly large hardened tumours can be felt in various situations in the abdomen, corresponding to the contracted intestinal walls. Shifting tumours, then, may be regarded rather as a diagnostic sign of the acute forms of lead colic.
The colic rarely commences without some slight prodromal symptoms of dyspepsia or gastric discomfort; generally for two or three days preceding the attack there is loss of appetite, with a distaste for food, and obstinate constipation, particularly a general feeling of languor associated with an unpleasant taste in the mouth. Tanquerel, and later Grissolle[3], among others, described a form of stomatitis which they thought was a prodromal symptom of an attack of lead colic. Our own experience, however, does not at all coincide with these statements.
[119]
Very occasionally the sufferer from acute colic may die during a paroxysm due to heart failure, but we have had no experience of such a fatality, although such an occurrence has been recorded on more than one occasion.
After the first acute attack of colic, which generally commences suddenly, often without previous warning, but is as a rule ushered in by irregular and finally complete constipation, or with diarrhœa alternating with constipation, the colic still occurs at irregular intervals; and although the constipation be relieved by enemata or the use of strong purgatives, paroxysmal pain will recur for days, and even weeks. In one particular case colic recurred at intervals for eight weeks, although the bowels were open each day and the patient had been under regular treatment, whilst the anæmia and other general symptoms of poisoning had disappeared.
According to the researches of Meillère[4] and others, lead is stored up in the body in various situations, and is gradually eliminated, such elimination taking place mainly through the fæces, and only to a limited extent through the urine. Probably the elimination of lead through the lower part of the intestine accounts for the recurrent attacks of colic.
Amino[5], Chatin[6], and Harnack[7], regard this colic as due to vaso-constriction taking place in the splanchnic area, and the rapid action of such drugs as atropin, chloroform, and nitroglycerine, support this view. In fact, Mayer[8] in 1881 demonstrated that in lead colic the splanchnic vessels undergo well-marked minute inflammatory changes. Others, from investigations carried on in persons who had died of lead poisoning, regard the acute pain as set up by irritation of the sympathetic nervous system, particularly of the solar plexus, irritation of the nerves in this region presumably setting up reflex colic.
The inhalation of amyl nitrite during an attack of colic will often entirely relieve it, and the pulse will immediately rise to the normal rate. It is difficult, however, in observing a case of colic, to determine whether the colic is preceded by slowing of the pulse and the rise of blood-pressure, or whether the colic is the immediate exciting cause of the constriction of the vessels and the alteration of the pulse-rate.
—The acute form of lead colic frequently passes on to a chronic condition; the attacks become much less intense, and may at times only amount to general discomfort in the abdomen,[120] but the symptoms may last for several weeks, and even months, with no abdominal discomfort for a period of a week or ten days, then recurrence of pain, gradually increasing until it has attained a considerable degree of intensity, and then passing away, only to reappear in two or three days’ time. In such cases of prolonged colic after an interval of two or three weeks, small doses of strychnine or tincture of nux vomica will determine the onset of an attack of colic, showing that the intestinal muscular tissue remains in a state of hypersensibility long after the attack appears to have passed away.
A particular form of colic of long duration with exacerbations and remissions has been known for many years in the French navy, called “seamen’s colic.” Before this time outbreaks had occurred in various parts of the world, and John Hunter[9] described a form of dry bellyache occasioned by drinking certain West Indian wines, the wines in question having been stored in contact with lead—in fact, the vigorous Saxon of John Hunter peculiarly describes this chronic form of lead colic.
Although the prodromal stages of malaise, lassitude, loss of appetite, nausea, etc., generally precede both the acute and the chronic forms, colic often commences suddenly. Men may be examined in the factory in the morning, when the ordinary routine examination has elicited no symptoms, and yet cases of acute colic have occurred later in the same day in the very men examined.
The chief points associated with lead colic are—
1. The intermittent character.
2. The relation of the colic mainly to the lower part of the abdomen.
3. The slowing of the pulse.
4. The relief afforded by firm pressure on the abdomen.
To which may be added the action of amyl nitrite and other drugs of a similar physiological action.
—Persistent headache is another of the symptoms associated with lead poisoning, but it is not common as an early symptom. The headache complained of by painters is probably not due to lead poisoning, but, as has been suggested, to turpentine. The headache of lead poisoning is invariably a later symptom, and frequently follows an attack of colic a week or more after the abdominal pain has ceased. The position of the headache varies; it may be of the vertex type, almost entirely[121] confined to the vertex and occipital regions. On the other hand, it is frequently irregular, and neuralgic in type; but in this type, frontal and temporal, more particularly temporal, the patient describes the pain as if a blunt instrument were being pushed through his head from both temporal regions at the same time. Earache, or pain in the region of the petrous portion of the temporal bone, may at times suggest ear disease, but this situation is not so common as suboccipital or temporal pain.
The headache in these situations is no doubt associated with the meningeal artery in the temporal region, and with the sinuses in the occipital region. The headache, not unlike the colic, undergoes remissions and exacerbations. With the exacerbations vertigo is common, and on more than one occasion in our experience a person suffering from persistent lead headache and vertigo has been arrested as suspect of alcoholism. Headache and vertigo without either colic or paresis is by no means uncommon, and may be associated with pains in the arms and legs. These pains are generally referred to by the patient as rheumatic, and it is a little interesting to call to mind the number of instances in which rheumatic symptoms are returned as associated with lead poisoning in the statistics given on p. 48. It is probable that these pains are neither muscular nor purely nervous in origin, but are primarily due to small lesions of the bloodvessels, as described in the chapter on Pathology, occurring in various parts of the body, and thereby setting up localized irritation, too minute to form an area which can be discovered by palpation, but sufficiently pronounced to produce irritation and reflex pain, in some respects similar to “bends” in compressed air disease. This special type of rheumatic pain differs, of course, from the lumbago associated with constipation.
Persistent headache is an exceedingly grave feature, and although it may at times disappear quickly on treatment, mental clouding and alteration of the higher functions is always to be feared; not infrequently persistent headache ushers in a final and fatal encephalopathy. In such a case the headache persists, becomes more and more excruciating, the patient rapidly shows loss of mental power, and may gradually sink into a condition of delirium. On the other hand, an attack of acute delirium may suddenly supervene, commencing with sudden loss of consciousness, followed by irregular movements of all the limbs, frothing at the mouth and nose, and finally mania. Recovery[122] is by no means uncommon, and after a sudden attack of this description the patients are entirely ignorant of the whole circumstance; they may occasionally recover powers of locomotion, and wander to long distances, unable to give an account of themselves or to remember their names, and only after a considerable time recover consciousness of their identity; but this type of case is comparatively rare.
The case quoted by Mott[10] gives a typical history of mental affection due, no doubt, to lead, but partially complicated by alcohol.
—Much controversy has raged around the significance of the blue line on the gums to be seen in certain persons working in lead, as to whether this particularly well-marked sign is to be regarded as a diagnostic symptom of lead poisoning or not.
For a long time it was regarded, and by many is still regarded, as sufficient evidence in itself to determine that a person is suffering from lead poisoning. On the other hand, those who have had considerable experience of industrial lead poisoning, particularly in the routine examination of workmen occupied in various lead industries, do not regard the occurrence of the Burtonian line as of more value than that the person showing such pigmented gums has been exposed to lead absorption.
There are two kinds of Burtonian line:
1. A fine bluish line is seen around the gingival margins, more pronounced on the interdental papillæ of the gum, and always more marked around such teeth as are coated with a deposit of tartar than around teeth which are clean. This line is undoubtedly due to the decomposition of the lead salts which have gained access to the mouth, by the sulphuretted hydrogen produced by the decomposition and putrefaction of food, epithelial débris, and other materials, which have accumulated around the edges of the teeth and in the interdental spaces. Peculiar evidence of this may often be seen in the mouths of certain persons whose parotid glands are discharging saliva which promotes deposits of calculus. Thus, one may often find merely the two first upper molar teeth on both sides of the upper jaw coated with tartar, no other teeth in the upper jaw being similarly affected. This deposit of calcium phosphate and carbonate is exceedingly porous, and becomes saturated with the products of decomposition, evolving sulphuretted[123] hydrogen in fairly large quantities. In the mouths of such persons working in lead factories a dark bluish staining of the cheek in apposition to the filthy tooth may be frequently seen, and where the rest of the teeth are free from deposit no such staining is observable. Viewed with a hand-lens, the blue line is seen to be made up of a large number of minute granules of dark colour which are deposited, often deeply, in the tissue. It is a matter of importance to note that a blue line is rarely seen in the mouths of those persons who pay attention to dental hygiene; where the teeth are clean, the gums closely adherent to the teeth, and entire absence of pus and freedom from deposit we have never seen a Burtonian line produced. Many of the so-called healthy mouths with perfect teeth have yet infected gums.
On examining sections of such a line, it is interesting to note that at first sight the particles appear to be situated deeply in the tissue, and mainly in relation to the bloodvessels supplying the gum. A little closer attention shows that the particles are really aggregated, particularly in the deficiencies between the epithelial cells which are constantly thrown off from the surface of the gum, a process which has its origin in an inflammatory condition, the whole gum becoming hypertrophied, with numerous small areas of ulceration. In these positions a certain amount of direct absorption of dust and fine particles takes place from the ulcerated surface, and becomes converted into lead sulphide by the sulphuretted hydrogen produced locally from the decomposing tissue. A certain amount of pigmentation is also referable to the mucous glands. It is well known that, in infections of the mouth of the type of pyorrhœa alveolaris or of rarefaction of the alveolar process, a good deal of infection coexists in the mucous glands of the buccal membrane, especially along the gum margins themselves, and the lead dust also becomes deposited in these glands, and later forms a sulphide. It is possible that some blue lines are due to excretion of lead from the blood.
Some little care is required to differentiate between the early lead Burtonian line and the curious bluish-grey appearance of the gum edges in cases of pyorrhœa alveolaris; when once the two conditions have been studied, little difficulty exists, but the use of a hand-lens will at once settle the matter. The bluish appearance of the gum in many cases of gum disease is due to local cyanosis. A few other forms of pigmentation of the gum[124] edges exist, such as an occasional blue line seen in workers with mercury, a black line in coal-miners, and so on, but these hardly call for discussion in the present instance. Any pigmentation of the nature discussed above is to be regarded as a sign that the worker has been subjected to the inhalation of lead dust, and is therefore suspect of lead absorption, in whom definite symptoms of lead poisoning may be expected to occur if the exposure to the harmful influence be long-continued.
2. In the second variety of blue line the pigmentation is not confined to the gum edge or to a band rarely exceeding a millimetre in width, as is the ordinary common blue line. In this case the whole of the gingival mucous membrane from the edges of the teeth, and extending some way into the buccal sulcus, five or six millimetres or even a centimetre wide, may be seen.
When this phenomenon is present, it is always associated with a marked degree of pyorrhœa alveolaris, the gums are soft, œdematous, and pus oozes from their edges, the teeth are frequently loose, and the other symptoms of disease of the os marginum are present.
Sections made from such a case suggest still more that some excretion of lead has taken place from the bloodvessels, as the lead particles may be seen closely related to the capillaries; but here again there is little doubt that it is due to absorption from the externally inflamed surfaces of the gum rather than excretion of the vessels themselves. It is interesting to note that, in all the experimental animals, in no instance has any Burtonian line been observed, although the animals (cats and dogs) have been fed upon cat’s meat, which readily undergoes putrefaction, and organisms capable of producing sulphuretted hydrogen are invariably present in the mouths of such animals. Notwithstanding this, the blue line has not been observed, because the animals’ gums were entirely free from infection or pathological changes. By causing an artificial inflammation around the canine teeth of an animal exposed to lead infection, a definite blue line was produced in two weeks. This line had all the characteristics of the common Burtonian line.
This form of blue line with a deep pigmentation of the whole of the gums, although in itself not to be regarded as diagnostic of lead poisoning alone, rarely occurs unless the person has been subjected to such long-continued poisoning that other symptoms have already made their appearance.
[125]
The blue line, then, whichever type is observed, cannot in our opinion be regarded as a diagnostic sign of lead poisoning, but is merely an indication that the person who exhibits the phenomenon has been at some time or other subjected to lead absorption.
There is no evidence to show that lead is excreted by the salivary glands. A number of cases of poisoning certainly complain of a metallic taste in the mouth, and, judging from the analogy of mercury, it is possible that excretion of small quantities of lead may take place through the saliva; but such a point is merely of scientific interest, and has no practical bearing on the question of lead poisoning. Pigmentation in the salivary glands suggesting excretion of lead has not been observed, notwithstanding the constant presence of potassium sulphocyanide in the parotid saliva. The blue line of Burton may occasionally be observed, in other regions of the body. From time to time the intestine is found stained with a bluish-black deposit of lead sulphide, and in a case of acute poisoning following the ingestion of a large quantity of lead acetate, and in the cases described by Oliver of the ingestion of lead oxide (litharge), black staining of the intestines was peculiarly well marked. In all the animals referred to it forms a constant feature in the large intestine, and in the chapter on Pathology this blue staining of the large intestine is more minutely described. We have met with it once in the post-mortem examination of a man who died of lead poisoning, and when found it may, we think, be regarded as a diagnostic sign. Macroscopical evidence is not sufficient; it is necessary to make a histological examination of the tissues, when the stained areas are seen to be associated with the lymphoid tissue in the intestinal wall, and not only interstitial portions, but actually the interior of the cells themselves, are found to be packed with small bluish granules. Such a histological finding would be highly characteristic of an extreme case of lead poisoning.
Where considerable quantities of lead have been taken into the gastro-intestinal canal, a blue ring has occasionally been described surrounding the anus.
About 85 per cent. of cases of lead poisoning with colic show obstinate constipation as a leading symptom. The constipation generally exists for several days preceding the onset of the colic, and may persist for as long as twelve to fourteen days,[126] while six to seven days is a common period. There is very little that is characteristic about the constipation other than its intractability; indeed, it is frequently of the greatest difficulty to relieve this symptom. No doubt the direct origin is due to the excretion of lead into the large intestine (see p. 94).
Palpation of the colon often shows distension, with a good deal of pain on pressure at both the hepatic and splanchnic flexures, more particularly the latter. Distinctly painful spots may be found in the length of the intestine, due to small ulcers, or more probably to the minute hæmorrhages which we have elsewhere described as associated with lead poisoning. The remaining 15 per cent. of cases have as a prodromal symptom diarrhœa. Further, diarrhœa is not uncommon amongst persons who are working in a lead factory, and who do not show other signs of poisoning; and as lead taken into the body in various ways is excreted through the fæces in common with other heavy metals, such as iron, bismuth, nickel, as well as arsenic, the occurrence of diarrhœa should suggest to the surgeon the possibility of considerable lead absorption having taken place.
[1] Zinn: Berl. Klin. Woch., No. 50, 1899.
[2] Collis, E. L.: Special Report on Dangerous or Injurious Processes in the Smelting of Materials containing Lead, p. 6. 1910.
[3] Grissolle: Thèse, Paris, 1835.
[4] Meillère, G.: Le Saturnisme, p. 122. Paris, 1903.
[5] Amino: Archiv. Ital. di Clin. Med., 1893.
[6] Chatin: Province Méd., Lyon, No. 4, 1892.
[7] Harnack: Deutsch. Med. Woch., 1897.
[8] Mayer: Virchow’s Archiv, 1881.
[9] Hunter, John: Observations of the Diseases of the Army in Jamaica. London, 1788.
[10] Mott, F.: Archives of Neurology and Psychiatry, vol. iv., p. 117.
[127]
—The two chief channels for excretion of lead are the urine and the fæces, while some include the saliva and the sweat.
In the case of the sweat there is not much evidence, but a few observers, mainly French, claim to have discovered traces of lead in the skin of lead-workers. In such a case, however, it is exceedingly difficult to eliminate the question of surface contamination; and although brisk peripheral circulation and transudation may possibly carry off a certain amount of lead, the chance of this is highly improbable.
There seems rather more evidence that the salivary glands may eliminate lead, as a number of other substances are undoubtedly passed in this way. Mercury certainly undergoes excretion through the salivary glands and the mucous glands of the mouth, and it is therefore not improbable that a metal so closely related in its chemical, and perhaps physiological, relations may be excreted in a similar fashion. Meillère[1] cites three instances of parotitis which were considered to be of lead origin, and further quotes an instance where, in chemical examination of the salivary glands after death, lead was found in small quantities.
Chronic parotitis is not infrequently cited as a symptom in cases of reported industrial lead poisoning, and may owe its origin to impairment of the salivary gland by the passage of the metal. Chronic parotitis, or even tenderness of the parotid glands, does not occur frequently among lead-workers with symptoms of lead absorption. Excretion, however, of lead through the salivary glands is not of great importance, except from the occasional complaint of a metallic taste in the mouth in chronic[128] lead poisoning, and in such instances possibly definite excretion of lead is taking place through the parotid glands. A case may be cited which rather supports the view that lead may be excreted through the salivary glands. A certain worker engaged in a dangerous lead process from time to time, but never as a constant symptom, showed distinct pigmentation in the internal surfaces of both cheeks in the region of the buccal papillæ of the parotid duct. The pigmentation was intermittent; at times a large patch of deep blue-black pigmentation was found in the situation on both sides, with no staining of the cheeks around or of the gum margins, although his teeth in these regions were coated with foul tartar. If the lead in this instance gained access through the mouth, why should it have been deposited merely upon the cheek in this one situation, despite the fact that several other situations in the mouth exhibited the same conditions of bacterial decomposition for the production of sulphuretted hydrogen? We have not observed this pigmentation in the neighbourhood of the ducts of the submaxillary and sublingual glands, but only in the parotid.
By far the most important organ in the excretion of lead, from the point of view of symptomatology and diagnosis, is the kidney. Lead is not uncommonly found in the urine of lead-workers and in the urine of those suffering from lead poisoning. The quantity present is usually small and in a form in which it is exceedingly difficult to detect. Yet very pronounced changes in the kidneys may take place, with little evidence in the urine itself that pathological changes are taking place.
The urine of workers in lead factories is frequently high-coloured; in fact, as a general rule the degree of pigmentation is greater than is normal, and in those persons who show some degree of icterus, with the curious yellowish-brown colour of the conjunctivæ, hæmatoporphyrin may be detected on applying suitable tests.
In well-established cases of chronic poisoning, albuminuria as a rule is found, together with certain alterations in the other constituents of the urine, such alteration frequently making its appearance before the definite onset of albuminuria. Further, the changes in the eye, referred to under a special heading, have been frequently described as albuminuric retinitis of lead origin, it being true that eye changes are often associated with chronic changes in the kidney.
[129]
In acute poisoning lead is generally found in the urine, but in chronic poisoning it is by no means a common occurrence. From time to time small quantities are excreted, and in the chemical analysis made of the kidneys in cases of fatal lead poisoning a certain amount of lead has frequently been noted. Wynter Blyth[2] found in the kidneys of two white lead workers a total of 0·003 gramme. Peyrusson and Pillault[3], quoted by Meillère, found a similar quantity, 0·003 gramme, while in experimental animals Meillère himself found considerably less, only 0·0001 gramme; Stevenson, in a case reported by Newton Pitt[4], 0·0086 per cent. of lead in the cæcum and colon. Notwithstanding a small quantity of lead which may be determined by chemical methods as present in the urine or the kidney, very definite nephritis is set up in these organs, obviously due to the irritative effect of the metallic poison.
—Kidney disease of several types has been described as associated with poisoning by lead, particularly with chronic lead poisoning, where large quantities of a soluble lead salt have been ingested. Very considerable strain is thrown upon the kidney, with the result that the lead salts themselves are passed through; but, as has been pointed out when dealing with acute poisoning, the passage of lead through the kidneys does not continue for any considerable time, and in lead poisoning of an industrial and chronic nature no lead at all has been found in the urine in undoubted cases. Even when present, it may be difficult to detect unless the electrolytic method is used (see p. 174). At the same time kidney disease undoubtedly does occur in a very large number of workers.
All heavy metals, of which silver, mercury, iron, zinc, and finally lead, may be quoted as examples, appear to be eliminated by the kidney when they are present in the body in toxic doses, and often when in small non-toxic doses, but in the latter case to a greater extent through the bowel than through the kidney. The lead circulating in the blood, in common with other heavy metals, may be found chemically in the kidney, but the quantity recovered is not as large as one would expect from the considerable amount of inflammation often present.
In the experiments on animals, subjected to poisoning over considerable periods, the condition of the kidney in every instance showed distinct histological changes; and the longer such animals had been subjected to the poisonous effects of the[130] metal, the more advanced were the signs of degeneration in its structure. In the earliest cases the disease partook much more of the nature of an interstitial nephritis, and it was in the later and more chronic stages only that changes in the glomeruli and fibroid degeneration were to be found, but in even these earliest cases of poisoning definite minute interstitial hæmorrhages were to be found scattered about the kidney. These minute hæmorrhages did not cause symptoms of hæmaturia, as in none of the experimental animals was bloody urine observed. On the other hand, besides definite small areas of hæmorrhage, patches were discoverable, indicative of hæmorrhage which had undergone fibroid change. Even in the illustrations given by Glibert[5], there appears to be evidence that hæmorrhage had taken place and had undergone fibroid degeneration, and there is very little doubt that in cases of kidney disease, the preliminary action of the poison determines small local yieldings of the vessel walls, with leakage, often hardly amounting to true hæmorrhage, at such spots. There is nothing opposed to the theory in the findings of other observers; in fact, if the preliminary gross effect be leakage of the description given, all the other lesions described by various observers follow as a corollary.
In the kidney, as in the other regions of the body, the venioles rather than the arterioles appear to be the preliminary site of destruction, and microscopical observation of the sections leads to the view that the intima of the vessels and not the media or the muscular coat is the one affected in the first place. Capillary hæmorrhages under these conditions are easier to understand than if the arterioles themselves or their muscular or middle coats were primarily affected. As degenerative changes progress, the whole of the vessel—external and middle coats and intima—undergoes change, ultimately resulting in the extreme narrowing and consequent blocking of the vessels themselves. Further shrinkage taking place in this area produces the shrunken sclerosed kidney.
Zinc in the form of oxide behaves in very much the same way upon the kidney as does lead. An experimental animal, which was given 0·2 gramme of zinc oxide per kilogramme body weight by hypodermic injection in the muscles of the back, died in fifteen days, and the kidneys showed extensive hæmorrhages—not merely the minute and capillary hæmorrhages found in lead[131] poisoning, but hæmorrhages extending right through from the cortex.
Clinically, kidney disease, unless albumin be detected in the urine, is not a prominent symptom during the progress of an attack of chronic lead poisoning, and is to be regarded as a late symptom, developing as the result of long-continued irritation. The difficulty of eliminating alcoholic complication has been discussed, and there are no specific symptoms or post-mortem signs which enable one to distinguish alcoholic nephritis from the nephritis of lead poisoning.
In the chapter on Pathology, the effect of alcohol on the kidney was cited as a common predisposing cause of kidney disease in lead-workers, and the effect of alcoholic excess in the case of a person who is already the subject of chronic lead absorption may determine the change from absorption to definite poisoning, because of alteration in the excreting-power of the kidney. So long as the ratio between ingestion and excretion is maintained the balance is kept up, and, although the tissues of the body may show signs of a certain amount of degeneration, no definite disease is produced; but the gastric irritation and the work thrown upon the kidney in removing from the blood large quantities of alcohol may be sufficient to alter this absorption-excretory balance and determine the attack of poisoning.
Acute nephritis is rare, and cannot be regarded as a sequela of chronic lead poisoning. Acute nephritis occurring in a lead-worker, with the associated symptoms of general œdema of the face, eyes, hands, feet, is of the gravest possible moment, and such a sudden appearance of nephritis is almost invariably fatal. In chronic nephritis, to which most of the lead cases belong, the usual signs are to be found in the urine. Pain is rarely a symptom; and although pain in the back is often complained of by lead-workers, examination rarely suggests that the backache is of kidney origin, but rather the lumbago type associated with chronic constipation. Care, however, must be taken, when backache is complained of, in eliminating kidney disease as a possible origin of the pain. A quantitative examination of the urine, with reference to the total acidity and phosphate excretion, may assist; and although this is not possible in a routine examination of cases of lead absorption, it may be useful in cases of suspected poisoning, especially where there are evidences of a good deal of blood-destruction.
[132]
For convenience of description, it is better to consider the action of lead upon the blood under two headings:
1. That of the corpuscular and other changes.
2. The action on the vessel walls, and pathological changes secondary to disease of the vessels.
—From the early days of medicine it has been known that lead produces poverty of the blood, and the white or yellowish-white appearance of persons who have been subjected to long-continued inhalation of lead dust or fumes constitutes striking evidence of blood-alteration. At the same time it is a common fact that the facial pallor does not always go hand in hand with diminution in the hæmoglobin. The conjunctiva may be observed as a test for colour, and here may be seen the curious vaso-motor disturbances which are partially responsible for the facial pallor.
Facial pallor in some forms of lead cachexia owes its origin to interference with the nerve-supply to the vessel walls, and it is a noticeable fact that a lead-worker whose face shows unmistakable signs of pallor rapidly flushes when spoken to suddenly or if mentally disturbed. The anæmia of lead poisoning is, however, a very definite fact. All observers are agreed that a marked diminution in the hæmoglobin of the blood takes place, to as low, often, as 35 per cent., without necessitating abstention from work, and without any serious interference with respiration, even when performing heavy manual labour.
In persons in whom the hæmoglobin is diminished there is frequently a yellowish or icterous hue of the skin, particularly the conjunctiva, due to staining of the tissues with altered blood-pigment. The hæmoglobin derivative—hæmatoporphyrin—can also be found in the urine of persons suffering from a marked degree of reduction in the blood-pigment, and may be taken as confirmatory evidence of destructive or hæmolytic anæmia. The symptom is, however, a later one than is frequently stated by the French observers, and can only be regarded as a later and confirmatory symptom, and not as an early one of diagnostic importance.
As would be expected from the destruction of blood-pigment, the morbid process leaves its imprint upon the individual red cells, and basophile staining of a number of the red corpuscles is to be found in a very large proportion of persons[133] poisoned by lead. Moritz[6] first pointed out that these alterations in the red cells were present in lead poisoning. The basophile granules are by no means confined to lead anæmia, but are to be found in any severe secondary anæmia where hæmolysis has taken place, as in nitrobenzene and aniline poisoning, carbon bisulphide, etc. In addition to the basophile-staining granules in the blood-cells, the whole corpuscle may take on a bluish-grey tint when stained. The best stain to demonstrate these bodies is Leishman’s modification of Romanowski’s, and there is no occasion to stain the blood in the fresh condition, as the presence of the granules may be demonstrated easily, even after two or three months. Schmidt[7] thinks that if the basophile corpuscles reach 100 per million red cells the cause is undoubtedly lead poisoning.
Alterations are also to be observed in the structure of the red blood-cells in addition to the basophile staining. Distinct vacuoles appear, but as a general observation—first noted by Glibert[8]—the blood appears to be more resistant to damage when making the films, and the red corpuscles themselves seem to be more elastic than normal (increased viscosity). Alteration in the shapes of the corpuscles also takes place, and not only small varieties—microcytes—but also the large macrocytes are to be found. Nucleated red cells are rare.
The diminution in the number of the red cells is not so pronounced as would be supposed from the diminution in the quantity of hæmoglobin; but in the later stages, as in other secondary anæmias of toxic origin, the total quantity of red cells sinks to a count of a million or less per cubic millimetre.
According to Garrod, etc., the alkalinity of the blood is decreased in lead absorption.
The white blood-corpuscles do not show any change in their structure by the ordinary methods of staining, but they apparently show, as do the red cells, more resistance to injury in spreading blood-films—that is to say, the viscosity of the blood, which is apparently increased in lead poisoning, is also exhibited by the white cells. In the early stages of lead poisoning, more especially in acute lead poisoning, distinct leucocytosis may be observed, such a leucocytosis showing itself rather in relation to the lymphocytes than to the polymorphonuclear cells. In addition, the large mononuclear cells are also greatly increased, and a differential blood-count from a case of lead poisoning which[134] also shows the presence of basophile granules in the cells invariably brings to light a definite increase in the percentage number of lymphocytes, and a decrease in the number of polymorphonuclears, and this even when the total leucocyte count is not outside the ordinary limit of normal variation. On the whole, the number of leucocytes present in the blood of persons suffering from lead absorption will always be found to tend rather towards the higher than the lower limit of normal variation.
At times a considerable increase in the number of eosinophile cells is found in films made from persons suffering from lead poisoning, particularly when there has been prolonged obstinate constipation. The count is never high, and is rarely more than 5 or 6 per cent. It is not usual to find any of the other forms of white cells in the blood, and in this way the anæmia of lead poisoning may be easily differentiated from the other forms, such as pernicious anæmia, lymphatic leucæmia, spleno-medullary lymphocythæmia. By examining a number of blood-films derived from persons subjected to lead absorption, and shuffled with a number of films from normal persons, one of us (K. W. G.) has been able to separate out, by the above method, the blood-films taken from the suspected persons. The criteria in the determination were—
1. The presence of basophile granules.
2. Total basophile staining and size of corpuscles—poikilocytosis.
3. Differential count, showing increase in a number of lymphocytes and large mononuclear cells.
Determination of the presence of lead poisoning from the examination of the blood, therefore, receives considerable support; but at the same time it is open to some objection from the fact that it is not in lead poisoning alone that basophile granules make their appearance in the blood. Any cause producing destruction of the red blood-cells, and even their depletion by prolonged hæmorrhage, is followed by an increase in the output of the red cells from the bone-marrow[9]. During this output of extra blood-cells from the bone-marrow, numerous cells gain entrance to the blood, in which the nuclei are not entirely degenerated; and it is these particular cells which give the phenomena of basophile staining, and their presence is rather indicative of the increased blood-formation that is progressing,[135] following blood-destruction, rather than direct evidence of blood-destruction itself.
In a number of forms of anæmia—in fact, in almost all forms of severe secondary anæmia, and certainly all forms of anæmia associated with hæmolysis—the presence of basophile granules may be demonstrated. They are commonly found in pernicious anæmia, secondary septic anæmia, and the anæmia of malaria. Practically, the use of basophile granules in the presence of the blood of lead-workers is being utilized in Leipzig for the early detection of lead poisoning. By means of the Zeiss eyepiece enumeration disc the relative number of basophile granule cells to normal red cells is determined; and when the number of red cells containing basophile granules exceeds 100 per million red cells, the individual from whom the blood is derived is regarded as in a presaturnine condition, and given proper treatment. By this means it has been found possible to diminish the number of persons actually suffering from lead poisoning.
The adoption of such a method has some drawbacks, especially in view of the fact that substances other than lead may cause the basophilia. At the same time there is no doubt that, if all persons employed in lead trades who showed basophilia were suspended from their employment at the present time, a very large number of persons would be dealt with. Yet the practical application of this method is by no means impossible under industrial conditions, and would at any rate give a definite test upon which diagnosis could be made, though it would be quite impossible to expect the general practitioner or the certifying surgeon to estimate the basophilic content. All such estimations would necessarily have to be performed at some properly equipped pathological laboratory, such as at the present time many municipal authorities possess.
These facts are of importance, as a differential count of the white cells, together with a careful inspection of a blood-film for basophile staining and alteration in the red cells, as well as other phenomena noted, together with an estimation of the hæmoglobin contained in the blood, are to our mind of considerably more value in the diagnosis of lead poisoning than is the quantitative estimation of the red or white cells. The following tables give a certain number of enumerations, etc., made from the blood of lead-poisoned persons:
[136]
BLOOD-EXAMINATION OF LEAD ANÆMIA—DIFFERENTIAL COUNT PER CENT.
[137]
The form of lead inhaled is immaterial, and definite poisoning with alteration in the blood may be occasioned, not only with white lead and lead fume, but also even with lead sulphate and lead silicate.
The following table gives the result of blood-counts performed upon the blood of persons employed in the manufacture of a paint erroneously supposed to be innocuous, as the base consisted of lead sulphate and oxysulphate:
Differential Counts per Cent. of Blood-Films from Lead Sulphate Workers.
| No. | A.[A] | B. | C. | D. | E. | F. | G. | H. | I. | J. | K. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 55 | 16 | 5 | 1 | 0 | + | + | - | + | + | + |
| 3 | 57 | 16 | 26 | 1 | 0 | + | + | - | - | + | - |
| 6 | 67 | 23 | 9 | 1 | 0 | + | + | + | + | + | + |
| 7 | 72 | 18 | 5 | 5 | 0 | + | - | - | - | + | + |
| 8 | 65 | 26 | 7 | 2 | 0 | + | + | - | - | + | + |
Sand-papering surfaces of painted objects, walls, coaches, etc., also throws a definite amount of lead dust into the air whenever the sand-papered paint contains lead. The following table gives the differential counts of the blood of persons employed in the furniture-painting trades:
Differential Counts per Cent. of Blood-Films from Furniture-Makers (Sand-Paperers).
| No. | A.[A] | B. | C. | D. | E. | F. | G. | H. | I. | J. | K. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 48 | 39 | 11 | 1 | 1 | + | + | + | + | + | + |
| 13 | 54 | 35 | 9 | 2 | 0 | + | + | + | + | + | - |
| 15 | 53 | 32 | 13 | 1 | 1 | + | - | - | - | - | - |
| 16 | 58 | 30 | 9 | 3 | 0 | + | - | - | + | - | - |
| 19 | 56 | 31 | 12 | 0 | 1 | + | - | - | + | + | - |
—A very large number of the symptoms referable to chronic and well-defined lead poisoning are referable to circulatory lesions, and, as has been elsewhere pointed out, the ultimate nerve degeneration occurring in various parts of[138] the body is probably but a final symptom of the earlier hæmorrhage which has taken place. Certain symptoms are, however, more closely related to the circulation than others, and may therefore be more conveniently grouped together under the present heading. The smaller changes, many of them connected with special organs (such, for instance, as the eye) or particular regions (as the mesenteric vessels), have been already referred to in dealing with colic and eye changes. Vaso-motor changes precede the actual change in the vessel walls themselves. On the other hand, the alterations in the structure of the liver, lung, spleen, and more especially the kidney, are secondary to change in the structure of the walls of the vessels themselves.
Vaso-motor disturbances may or may not be of nervous origin, although the former view is probably correct, and it is also equally possible that the direct affection of the vessels is associated with the nerve irritation. On the other hand, direct inflammation of the vessel walls, resulting in obliterative arteritis, in arterio-sclerosis, and degeneration and exudation in the kidney, lung, and liver, are practically due to degenerative changes either in the intima or the middle coats of the finer vessels. The common symptoms of arterio-sclerosis, vertigo, headache, and pulsation in the vessels, and of the persistent headache already referred to, all suggest changes taking place in the vessels complicated by œdema. In the early stages of kidney degeneration, however, it is common to find an interstitial nephritis due apparently to exudation from the vessel walls. Such an hypothesis is to some extent supported by the somewhat allied condition of engorgement and fibroid change in the liver and lung, and to a lesser extent in the spleen. In the lung, even in persons not exposed to inhalation of lead, and in animals, as pointed out by Glibert, secondary changes in the lung follow lead intoxication, such changes taking the form of emphysema and generalized fibrosis; whilst the liver is engorged with blood, and later undergoes similar degenerative changes. The bloodvessels in these organs are found to have lost a considerable amount of their elasticity, to have yielded here and there, and in other places to be completely closed by obliterative arteritis. Microscopical hæmorrhages are to be found mainly in the veins leading from the capillaries. In the kidney such vessel changes as are outlined are the precursors of disease, and albumin is found in the urine, but the quantity is rarely very large. Casts[139] are not common, and the amount of lead present in the urine may be exceedingly small, difficult to trace, and in many cases entirely absent.
In the later stages of chronic saturnism the heart may show degenerative changes. Microscopical examination of the heart muscle shows that alteration of the fibres of the muscles takes place in a manner similar to that of the voluntary muscles. Disease of the heart valves is uncommon; alterations in the heart sounds are infrequent; the clinical picture of the cardiac condition is that of a “flabby” heart.
[1] Meillère, G.: Le Saturnisme. Paris, 1903.
[2] Blyth, Wynter: Proc. Chem. Soc, 1887-88.
[3] Peyrusson and Pillault: Meillère’s Le Saturnisme, chap. iv.
[4] Newton Pitt: Trans. Path. Soc, No. 42, 1891.
[5] Glibert: Le Saturnisme Expérimental: Extrait des Rapports Ann. de l’Inspect. du Travail, Brussels, 1906.
[6] Moritz: Mediz. Woch., St. Petersburg, 1901.
[7] Schmidt: Arch. für Hygiene, vol. lxiii., p. 1, 1907.
[8] Glibert: Ibid.
[9] Boycott: Journal of Hygiene, 1910.
[140]
—The most definite objective symptoms of chronic poisoning by means of lead are those of the nervous system. From the time of Tanquerel[1] affections of the hands and fingers and the muscles of the back have all been well known. Associated with the paralysis are local vaso-motor effects, such as cyanosed condition of the skin over the paralyzed muscles, cold hands, etc.; whilst later, if the paralysis be severe and persists, atrophic changes take place in the skin, muscles, bone, whilst definite contracture occurs from unopposed contraction of the unaffected muscles. Paralysis will therefore be associated with two of the great systems into which the body is divided for the purposes of medical and physiological description—namely, the muscular and the nervous—and, on account of the similarity of the clinical symptoms of lead paralysis, attention has been drawn rather to the nerve changes preceding muscular paralysis and degeneration than to other influences affecting the nerve inflammation.
Lancereaux[2] considered that lead poisoning resulting in paralysis takes the form of a gradual impregnation of the nervous tissue with lead salts, until such a time as degenerative effects are set up, and with it muscular paralysis.
Meillère[3], who has given much attention to the ætiology of lead poisoning, as well as to the clinical study of the disease, considers that plumbism may be divided into three periods:
(a) Impregnation of the tissues of the body, the nervous tissue being the chief one affected by lead salts.
(b) Retardation of the general oxidation changes of the body, resulting in malnutrition and general loss of tone.
[141]
(c) Establishment of intoxication, with the generalized affections, paresis, etc.
If three such periods can be recognized, as no doubt they can, as divisions of the time during which lead gradually affects the tissues, the symptoms in the more severe cases would be expected to be those associated with the more prolonged exposure. This, no doubt, is true to a limited extent, more particularly in industrial poisoning, depending for its development on a long-continued dosage of lead in minute quantities, and for the most part of metallic lead. On the other hand, with some of the salts of lead, notably the hydrated carbonate, acute disease may take place during the first stage—namely, impregnation of lead—the determining factor then being either the retardation of the elimination of lead, or a suddenly increased quantity in lead dosage, or some intercurrent disease, or even alcoholic excess, whereby a sudden large excess of the poison is thrown into the general circulation.
The commonest type of paralysis occurring is the one affecting the muscles of the hands, which may for a considerable time show some diminution in their extensor power before the actual onset of the disease takes place. The onset of paralysis is practically always unaccompanied by pyrexia; the only occasions in which pyrexia may be associated with the onset of the attack are those cases in which some secondary cause determines the paralysis, and the pyrexia in these instances is due to the intercurrent disease, and not to the lead infection.
Although weakness of the extensor muscles of the hands may be present in persons subjected to lead absorption for a considerable time, the actual onset of the disease itself is frequently sudden; but in the majority of cases it is distinctly chronic, and is rarely, in the case of paresis, associated with any definite prodromal symptoms. Prodromal symptoms have been noted, such, for instance, as lassitude, general debility, and more especially loss of weight. Cramps of the muscles the nerve-supply of which is becoming affected, alteration of the skin over areas corresponding to definite cutaneous nerves, hyperæsthesia, anæsthesia, or analgesia, may occasionally be present. Neuralgic pains have also been described, but these are inconstant, and generally of the arthralgic type related to the periarticular tissues of joints rather than to pain in the course of the nerves. The pain is rather of the visceral referred type[142] than a definite neuralgia. Tremor is, however, frequently associated with the preliminary condition of paresis, and in several cases variations in the amount of weakness of the extensor muscles of the wrist have been noticed, as far as can be estimated clinically without the use of a dynamometer. Associated with this weakness is tremor of a fine type, often increased by movement (intention tremor), and in every case more marked during the periods of increased weakness. Instances have occurred where definite wrist-drop followed after a prolonged period of weakness; in others the weakness has temporarily cleared up for six months, and no difference could be determined in the extensor power of the two wrists; while in others, again, the weakness is progressive, but slight, and insufficient to warrant the removal of the workman from his occupation. Again, progressive weakness may remain a symptom of the wrists of workmen for years.
—The paralysis may be partial or generalized, but the chief muscles affected are the extensors of the wrists and the forearm, and the interossei of the hand. As a rule, the first muscles to be affected are the extensor communis digitorum and the extensor indicis. The muscles of the shoulder—mainly the deltoid—come next in order, followed by the muscles of the leg, particularly the peroneus longus and brevis, with occasionally the interossei of the foot; the muscles of the back, neck, and abdominal walls, are occasionally affected, as are those of the larynx and diaphragm, and it is of interest to note that Trousseau pointed out that among horses employed in lead works paralysis of the superior laryngeal nerve often occurred.
Considerable difficulty is experienced in estimating the reason of the predilection of lead for the musculo-spiral nerve, this being the nerve mainly affected in wrist-drop. Owing to the fact that the supinator longus receives an additional nerve-supply to the musculo-spiral, this muscle frequently escapes paralysis when the whole of the other extensors of the hand are involved. Moreover, the predilection for given nerves differs in different animals, and one of us (K. W. G.) has found experimentally that in cats the first nerve to be affected is the anterior crural supplying the quadriceps extensor, whilst the second group of muscles affected are the spinal muscles, particularly in the lumbar region.
[143]
Among the speculations which have been made with regard to this predilection for definite groups of muscles supplied by one nerve, Teleky[4] examined forty cases of paralysis with special reference to Edinger’s theory—namely, that the function of muscles (and of other organs) breaks down under certain circumstances before the strain set upon them. In this way Edinger explains paralysis following lead poisoning as being due to excessive strain on the particular group of muscles affected, based on a consideration of the relative volumes and weights of the muscles of the hand and forearm, and the demands made on the several groups, flexors, extensors, supinators, etc., by the coarse or fine work respectively demanded of them in industrial employment. He concludes that—
1. Of the forearm, the flexors (triceps, anconeus, extensors, biceps, brachialis anticus, and supinator longus) possess a high degree of capacity for work, but are not called into play mainly in the execution of fine work; while the supinators are characterized by great mass, and are brought into play mainly in work of coarse and heavy nature, and not during fine manipulations.
2. The muscles concerned in pronation are of small capacity for work, and are not called on for sustained work.
As for the muscles acting on the wrist and hand, he concludes that the extensors (carpi radialis longior and brevior, carpi ulnaris, and the extensors of the fingers) are powerful, and much exceed in capacity for work the flexors (flexor carpi radialis, flexor carpi ulnaris, the flexors of the fingers, etc.), but in all fine manual work, and specially where close grasping movements enter into association with the flexors, external strain is put upon them, whilst the flexors merely support their action.
The extensor communis digitorum is the weakest of all the long finger muscles; its volume is hardly one-fourth that of the corresponding flexors, and while it acts only on the first of the phalanges, the flexors act on all three. In all fine work they are called on for heavy strain, especially the interossei and the lumbricals, but in harmony with the long flexors when grasping movements are performed. The small muscles of the fingers have nearly the same mass as the extensor communis, and in all fine movements the grasping efforts are taxed severely; but their play is under considerably more favourable physical relations than that of the extensors, whilst in addition they are aided in[144] their work at times by the long flexors. The chief adductor muscle of the thumb (extensor metacarpi pollicis) is particularly powerful; the other extensors of the thumb are very weak, and work under unfavourable physical conditions, but are supported in their action by the strong abductor muscle. The muscles of the abductor, opponens and flexor brevis, in the complicated work thrown on the thumb in manipulation, are much exerted, so that the effects of overexertion show themselves first in this region.
Thus, Edinger maintains that the muscle-supply of the arm is designed for coarse heavy work, the muscles of the fingers and the hand having to carry out more work than can be expected of them from a consideration of their volume and their physical action.
The commonest form of lead palsy, the antibrachial type of Déjerine-Klumpke[5], is explicable from consideration of overexertion of the particular group of muscles named. The supinator muscle, supplied also by the musculo-spiral nerve which serves the paralyzed muscles, escapes because of its size, and the fact that functionally it belongs to the flexor group, and the first-named reason also, explain the frequent escape of the long abductor of the thumb.
Teleky[6] investigated thirteen slight cases of the antibrachial type. In one both hands were affected, in one the left only, and in all the others the right only—facts which he thinks bring out the rule of causation by employment. Of fourteen painters, three had the right forearm affected only, the other eleven both right and left, but always more marked in the right. Amongst them he cites cases where the shoulder muscles were paralyzed, which he considered was due to the extra strain of unusual employment, involving a raising of the arms above the head, or lying on the back painting the under parts of carriages. In several of the painters the index-finger was the least affected, by reason of the less exertion thrown on it by the position assumed in holding the brush between the second and third fingers. The long abductor, probably because of its size and power, is in no case completely paralyzed.
In file-cutters he insists that the predominant share, falling on a single or on several small muscles of the hands, makes the early appearance of paralysis of the small muscles the characteristic sign. In this connection we have frequently observed[145] decrease in the size of the thenar and hypothenar eminences amongst lead rollers; in fact, in the majority of lead rollers who have followed their occupation for a large number of years the flattening of both thenar and hypothenar eminences is well marked, but it is only fair to point out that in these cases very considerable stress is thrown on the muscle of this part of the hand by the pressure of the lead plate in pushing it inwards into the roller and grasping it on its appearance back again through the roller, and that, further, the use of large and clumsy gloves with all the fingers inserted into one part, and the thumb only into the other, tends to produce inaction of portions of the lumbricals and of the opponens pollicis, and may, therefore, from purely mechanical reasons cause damage to this part of the hand.
Teleky[7] cites cases of right-sided paralysis of the adductor brevis pollicis supplied by the median nerve, and partial paralysis of the long extensors and of the extensor ossis metacarpi pollicis supplied by the radial nerve, and in one or two cases complete paralysis of the thenar muscles and adductor, whilst the extensors of the fingers and wrists were only partially paralyzed. The cases all occurred in lead capsule polishers. This particular selection of muscles is clearly the result of the peculiar movement necessary in polishing the capsules of bottles on a revolving spindle, involving specially the use of the opponens muscle.
This observation of Teleky’s is in direct accord with the observation quoted above of the hands of persons engaged in lead rolling.
In the lower extremity, paralysis of the muscles associated also with paralysis of the adductor and extensors of the thumb was found by Teleky in a shoemaker who had contracted plumbism by the use of white lead. He explains this lower extremity paralysis by the exertion thrown on the adductor muscles of the thigh whilst holding the shoes.
In children affected with lead palsy the lower extremities are more frequently paralyzed than the upper, due to the relatively greater strain in childhood on the legs than on the arms.
Edinger’s theory, supported as it is undoubtedly by Teleky’s observations, is a matter of the greatest importance in the production of paralysis; for if we accept the view that lead is a poison that has a selective power on certain nerves, we have still to consider which is the greatest force, the selection[146] of certain groups of muscles or the effect of functional action. The theory of muscular overexertion certainly falls in with the type of paralysis, and Teleky has undoubtedly shown that under certain circumstances, by special exertion of other muscle groups not usually affected, these muscles alone, or to a greater extent than those usually affected, are involved in the paresis. If, therefore, lead has a selective action, which is exceedingly doubtful, it must be very slight.
Such selective action is, of course, exceeded by a functional activity which brings about the affection of those nerves which supply the muscles most used. If, therefore, we judge of paralysis as being due to the selective action of lead on certain nerves, we are met at once with the objection that the muscles affected do not always correspond to such a nerve distribution, and that muscles supplied by other than the musculo-spiral nerve are affected by paralysis.
Careful consideration of the chapter on Pathology, and more particularly of the histological findings described, in which the preliminary action of lead is found to be typically and invariably on the blood, setting up degenerative changes microscopical in size and limited in area, affecting the vessel walls and producing a yielding of the vessel, determining minute microscopical hæmorrhages distributed, not necessarily in one position of the body, but all over the body, and peculiarly in the case of the cat affecting those muscles called upon to perform sudden and violent movement—namely, jumping—enables us to regard such microscopical hæmorrhages as an adequate explanation of the association of paralysis in muscle groups, functionally related to various trades and industrial processes.
It may be argued, and we think with considerable reason, both from pathological and clinical findings, that, as muscular exertion is apparently associated with the onset of paralysis, particularly in those muscles which may be regarded as physically somewhat inadequate to the work they have to perform, and that, as the paralysis is associated with definite functional groups of muscles, and to a curious extent varies according to the trade in which the sufferer is engaged, therefore greater stress thrown upon the muscular tissue at some period or another during occupation determines the microscopical hæmorrhage in the nerve supplying the muscles, or in the muscle itself, so that[147] the paralysis affects just such muscles as have an increased strain thrown upon them. It does not necessarily follow that the preliminary initial hæmorrhage occurring should be a large one—in fact, from the whole of the histological history, hæmorrhages are exceedingly minute; neither does it follow that it is essential for such hæmorrhages to take place in the whole length of the nerve itself; but it is only necessary that the finer branches of the nerve should have their venioles or arterioles affected, and it is of course in the finer branches particularly, as has been pointed out in relation to the venioles, that degeneration of the intima of the vessels takes place.
Finally, the effect of early treatment on lead palsy tends to bear out this theory. If a case of lead palsy be treated in the early stages, the clinical course of the case is good; increased paralysis generally takes place in the first week, and, where, perhaps, only two or three fingers are involved when the case is first seen, spreads generally to other regions within a week, and the whole hand is affected; but from this moment onwards improvement takes place on the application of suitable treatment, and, if continued, almost invariably results in the entire recovery from the paresis.
There is little doubt that this is the true explanation of the ordinary paresis of lead poisoning, and a very great deal more evidence is required to combat it and to prove the selective action of lead upon individual nerves, since the theory of hæmorrhage does not owe its origin to conjecture, but is based on clinical and histological examination of early cases of poisoning.
In attempting to find a cause for the paralysis of the hands so commonly present in painters, it has been suggested that lead is absorbed through the skin, and affects the nerves at their junction with the muscles, setting up a peripheral neuritis [Gombault[8]]. The theory breaks down at once when such commonly-occurring affections as paralysis of the ocular muscles, paralysis of the peroneal type, paralysis of the muscles of the shoulder, etc., are considered.
For the convenience of description lead paralyses are generally divided into a series of groups, the grouping varying according to the function of the various muscles rather than to their anatomical grouping. The various types of paralysis have to be considered in detail:
[148]
—The first muscle affected is the extensor communis digitorum, with dropping of middle and ring fingers, while extension of the first and fourth is possible because of their separate muscles of extension (extensor minimi digiti and extensor indicis). Paralysis may be limited to these, and not advance farther, but it is common to see these two muscles primarily affected, and for other muscles to become involved after the patient is put on treatment, although exposure to lead has ceased. Usually, however, the paralysis advances, involving the extensors of the index and little fingers, so that the basal phalanges of the four fingers cannot be extended. The long extensor of the thumb is next involved, but this may be delayed. The two terminal phalanges are still able to be extended by the interossei (as shown by Duchenne) when the basal phalanx is passively extended on the metacarpal. Abduction and adduction of the fingers also remain unaffected. The wrist muscles are affected next. The hand remains in semi-pronation, and when hanging down forms a right angle with the forearm, the fingers slightly flexed with the thumb towards the palm, and the hand deflected to the ulnar side. In grasping an object the flexors remain unaffected, the wrist is much flexed owing to the shortening of the flexors in consequence of the extensor paralysis. The hand cannot pass the median line. The long abductor of the thumb—that is, the extensor ossis metacarpi pollicis, also known as the “extensor primi internodii pollicis”—is only very rarely involved, but has been described as being the muscle alone involved in the paralysis affecting persons engaged in polishing lead capsules.
—The muscles affected are those of the Duchenne-Erb group—namely, the deltoid, biceps, brachialis anticus, and supinator longus. The supra- and infrascapular muscles are also as a rule involved, but the pectoralis major rarely. This type of paralysis is usually found in old cases associated with other forms of paralysis, but may be found as a primary affection (as already noted amongst painters); sometimes the deltoid is the only muscle affected, with diminution in electrical contractility of the other muscles of the group.
The arm hangs loosely by the trunk, with the forearm semi-pronated. The arm cannot be raised, nor can the forearm be[149] bent on the upper arm. Extension is unaffected, as the triceps is never involved. Supination is impossible because of paralysis of the supinator brevis. Movement effecting rotation of the shoulders is involved, due to the paralysis of the supra- and infra-spinatus. Electrical reactions are said to be less marked in the brachial than the antibrachial type, and complete loss of faradic contractility is rare; but in one of the three cases described below, in which electrical reactions were carefully tested, the right deltoid showed entire loss of contractility to faradism.
—The muscles of the thenar and hypothenar eminences and interossei are affected. This type of lead paralysis may be distinguished from progressive muscular atrophy by the electrical reactions, and the fact that the atrophy is accompanied by more or less pronounced muscular paralysis. The atrophy is almost always most marked, and advances pari passu with the paralysis. This form may occur alone, or be complicated with the antibrachial type, which is the most common. It is seen in file-cutters as the result of overstrain of the muscles in question. Moebius[9], in his observations on file-cutters, noted in one case paralysis of the left thumb, with integrity of the other muscles of the left upper extremity. Opposition of the thumb was very defective; there was paralysis of the short flexor and of the adductor and atrophy of the internal half of the hypothenar eminence. Reaction of degeneration was noted in the muscles named, but not in the extensors of the fingers and wrist. In another case, in addition to feebleness of the deltoid, flexors of forearm on the upper arm, and small muscles of hand, there was paralysis and atrophy of the adductor of the thumb and first interosseous and paralysis of the opponens.
—This is a rare type, and nearly always associated with the antibrachial or with generalized paralysis. In the former the paralysis is slight, especially when it affects the psoas; but there is predilection for certain groups of muscles, especially the peroneal and extensor of the toes, while the tibialis anticus escapes. Hyperæsthesia, or more rarely anæsthesia, precedes the onset.
The patient walks on the outside of the feet, has difficulty in climbing stairs, and cannot stand on the toes. The toes drag on the ground in walking, so that the foot has to be swung round[150] at each step, and the inner side is lifted in excess by the action of the tibialis anticus, with uncertainty in gait. If walking is continued, the toes drag more, and “stepping” gait is assumed by bringing into action the muscles of the thigh. The foot cannot be flexed on the leg; abduction of the foot and extension of the basal phalanx of the toes is impossible. Later the peroneal muscles, extensor communis of toes, and extensor of great toe, are paralyzed, from which fact arises the difficulty of walking and of descending stairs, as the whole weight of the body is supported then by the tibialis anticus. This type corresponds with the antibrachial type of the upper extremity. If the tibialis anticus is paralyzed, it is in association with the gastrocnemius.
—Tanquerel[10] called attention to the aphonia of horses in lead works, necessitating tracheotomy, and Sajous[11] described adductor paralysis of the glottis in a house-painter. Morell Mackenzie[12] also described unilateral paralysis of the adductors in persons suffering from lead poisoning, whilst Seifert[13] particularly describes a curious case in which paralysis of the transverse and oblique arytenoid muscles prevented contraction of the cord in its posterior quarter, and in a second case of paralysis, affecting the posterior crico-arytenoid muscles on both sides, the adductors remained unaffected. What is still more interesting in Seifert’s case is the fact that at the post-mortem old hæmorrhages were found in the mucosa of the arytenoids and aryepiglottidean folds. Occasionally sensory paralysis may be found in the special sense organs—such, for instance, as loss of taste, loss of smell, and, in addition, diminished power of hearing—but these defects rarely if ever occur unless accompanied by distinct mental changes and generalized paralysis.
—Poisoning affects the eye in two ways:
Lockhart Gibson[14] describes a large number of cases of paralysis of the muscles of the eye met with amongst children in Queensland. The cause was traced to the painted railings near which the children had been playing. The white lead paint had somewhat disintegrated under the action of the sun’s rays, forming an efflorescence; the children admitted to have rubbed the paint and then sucked their fingers.
[151]
Between July, 1905, and 1908, sixty-two cases of plumbism in children were admitted to the children’s hospital, and of these sixty-two cases thirteen had well-marked ocular symptoms. The paralysis of the muscles of the eye was almost invariably one of the external rectus, but other muscles were at the same time affected; occasionally paralysis of the whole of the oculo-motor muscles was seen with the exception of the superior oblique. It is worthy of note that amongst these children a very large number suffered, in addition to their eye paralysis, with foot-drop and wrist-drop, and, on the whole, suffered from foot-drop to a much larger extent than from paralysis of the hands.
Galezowski[15] describes paralysis of accommodation of the eye, and in cases described by Folker[16] some amount of orbital paralysis was also present.
—These types do not differ in form from the previously described types, except, perhaps, as regards their rapidity of onset. Where the onset is slow (chronic) the subject is usually one who has been previously affected with paralysis of the extensors of the hand, followed by development of the paralysis in the shoulders, hand, leg, thorax. In the acute form, paralysis may affect all the muscles in a given limb or group, and reduce them in a few days to a complete condition of paralysis. In extreme cases the patient lies on the back and is incapable of rising, and sometimes even unable to eat. The intercostals, diaphragm, and larynx, are also affected, while there is generally dyspnœa and aphonia. The muscles of the head and neck appear to escape. In these acute cases, pyrexia may be a common symptom.
—The diagnosis of the affected muscle is greatly assisted by careful examination of all the muscles in the affected physiological group by means of the galvanic and faradic currents. The battery for the purpose of testing the electrical reactions must have an available electromotive force of over 40 volts. A battery of thirty-two Leclanché dry cells is ample. For the faradic current, a small induction coil operated by two Leclanché cells is sufficient. One large, flat electrode should be used, and several smaller ones.
The faradic current should be used first, as it stimulates the nerves directly, and the muscles only indirectly, through their nerve-supply. Each nerve trunk should be examined systematically. The motor points correspond for the most part[152] with the points of entry of the motor nerves into the muscles which they supply. A small electrode, either a button or a small disc about the size of a sixpence, should be used for the examining electrode, while the larger electrode should be placed either on the abdomen or between the shoulders. The electrodes should be well soaked in normal saline.
The intensity of the minimum current required to produce a contraction for each point should be noted, and compared with the effect of a similar current on the opposite side of the body.
The reaction of degeneration of the faradic current consists in no contraction at all being elicited, even when a very strong current is employed. If there be unilateral wrist-drop in the left hand, consisting of loss of power of the extensor communis digitorum on that side, no movement of the muscle is produced at all when the electrode is placed across the motor points of the muscle. These are situated to the outer side of the arm when the dorsum of the hand is uppermost, about 1¹⁄₂ to 2 inches below the olecranon. The same quantity of current, when applied to the unaffected muscle on the opposite side, produces a brisk reaction.
Having observed the effect with the faradic current, and the results having been recorded, the continuous current is used, and the electrodes made use of in an exactly similar fashion. When a small electrode is used, the superficial nerves and muscles are more stimulated than those lying deeply. It is necessary, therefore, to begin with a small current and gradually increase it until the individual muscle responds.
The strength of the current employed is registered by means of a milliampèremeter.
With the continuous current quantitative as well as qualitative alterations may be determined, and with the quantitative change of the galvanic current the muscular excitability is increased, contraction following the application of a weaker current than is necessary to produce it in health or in the sound muscle on the other side of the body.
With the qualitative change, the contraction is no longer sharp, but sluggish. The anodal closing contraction is elicited with a weaker current than kathodal closing contraction, so that ACC>KCC.
The quantitative change depends partly on the nutrition of the muscle; the qualitative change depends on the fact that[153] the nerve no longer regulates the character of the contraction, and also to a small extent is the result of changes in the muscle itself.
In a complete reaction of degeneration in an affected muscle, reaction to the faradic current is absent, contraction to the galvanic current is sluggish, and is produced with a smaller current in the anode than the kathode.[A]
[A] The kathode, or negative electrode, is attached to the zinc rod; the anode, or positive electrode, to the copper or carbon.
As the nerve lesion passes away, the voluntary contraction generally begins to return before the nerves show any reaction to electrical stimulus.
The following three cases in which the electrical reactions of the muscles were determined, give examples of typical cases of lead paresis. In No. 3, owing to the fact that the case was treated immediately the paralysis occurred, complete recovery had taken place, and, as will be seen, the electrical reactions have again become normal.
Case 1.—Litharge and blast-furnace worker. Employed in lead works, where a large number of different metallurgical processes associated with the recovery of lead from the ore were carried on. Double wrist-drop, existing for eight years, untreated until some four years after paralysis took place, when slight improvement occurred. The electrical reactions show that the extensor communis digitorum on the right side is completely degenerated, whilst the first interosseous of the right side show reactions of degeneration. On the left side the extensor communis digitorum showed normal but very feeble reaction. This latter point is one of considerable importance if early hæmorrhage accounts for lead paralysis, for if the nerve itself was completely destroyed, or if, as was the case, the muscle appeared completely paralyzed on inspection, obviously the nerve-supply must be completely cut off if the lesion was due to destruction of the nerve of the spinal cord or to the destruction of the lower motor neuron. On the other hand, the presence of small localized fibrillar contraction, found by the galvanic current, together with the presence of a slight reaction to faradism, suggests that some small portion of the nerve has remained unaffected, and that for this reason certain portions of the muscle have not undergone degeneration—a circumstance which can hardly be expected if the cause of the paralysis is in the destruction of the whole of the nerve-supply.
[154]
[156]
This case is a typical one of the anterior brachial type, showing partial recovery of function. The man is able to grasp, although the wrist becomes strongly flexed in so doing.
Case 2.—We are indebted to Dr. Gossage for this case, which was presented at the Out-patients of the Westminster Hospital; and to Dr. Worrell, who made the electrical investigations. We are further indebted to Dr. Worrell for the reports which are given in tabular form of the electrical reactions of these three cases.
This is a case of brachial type, with weakness of both deltoids, and the patient was unable to raise his right arm at the shoulder. It will be seen that there is here also evidence that the electrical contractility diminishes before the entire loss. It will be also noticed that the supinators are unaffected.
Case 3.—These are the electrical reactions of a case which had recovered. This man showed the ordinary anterior brachial type, which came on suddenly, although he had shown distinct weakness of wrists when forcible flexion was performed for nine months previously, but there had been no obvious increase in the weakness. He was immediately removed from his work, and within seven days paralysis, which at first only affected the extensor communis digitorum, had spread to the minimi digiti and the extensor indicis, the opponens pollicis being also involved on the right side. He was treated from the start with faradic current, and was instructed to use the battery himself, which he did twice a day for a year. At the end of two months he was sufficiently recovered to be given light work, and at the time of taking the reactions his wrists have so far recovered their power that we were unable to flex them forcibly.
The progressive weakness noted in the three cases has already been referred to, and may be a prodrome of paralysis, but there may be recovery without paralysis supervening.
—Tremor may be observed in a large number of cases of lead poisoning, and is invariably associated with paralysis, although the symptom of tremor by no means always progresses to definite palsy. Two types of tremor are described—fine and coarse—and Gübler further describes a type of tremor which is both rhythmic and intermittent. The tremor is usually distinctly increased on attempting to grasp or point the hand (intention tremor), but it is difficult to separate tremor from alcoholic tremor, and, further, it must not be forgotten that[157] persons engaged in arduous work may show a certain amount of tremor due to muscular fatigue. Persistent tremor, however, is a symptom that is always to be noted and carefully watched.
Of the types of paralysis, the antibrachial is the most common, and, secondly, probably the brachial. The least common is the peroneal. The table on p. 54 shows the distribution of cases of paralysis, so far as they can be made out from reports received since 1904.
Closely associated with paralysis are affections involving the brain. Tanquerel, in his classical description of affections of the brain associated with lead poisoning, gives the following classification:
There is probably a very considerable relationship between insanity and lead poisoning, as pointed out by Robert Jones[17], the resident physician and Superintendent of Claybury Asylum.
Rayner[18] remarked that the compulsorily careful habits of life of painters and lead-workers ought to protect them against vicious habits, and should protect them against a too free indulgence in the use of alcohol! Our own experience is that paralysis, and particularly affections of the brain, occur in the majority of cases in persons who are addicted to alcohol, and the experiments quoted in the chapter on Pathology on the influence of alcohol in the production of encephalitis in animals is strong presumptive evidence that alcohol is one of the chief predisposing causes in the determination of saturnine encephalopathy.
Encephalitis is given as a cause of death in the report already referred to in 14·3 per cent. of 264 fatal cases. Amongst this number encephalopathy accounted for 14·3 per cent., cerebral hæmorrhage 9·8 per cent., and paralysis 9·2 per cent. Now, all these are cases in which cerebral lesions may be confidently stated to have existed, which brings the total to 34·4 per cent. of deaths at least due to brain involvement. We have already referred to the high incidence of paralysis amongst file-cutters—40 per cent. as against 21·1 per cent. for all industrial forms of poisoning.
[158]
When encephalitis occurs, it is usually an acute symptom, and often develops before paralysis is set up, but as a rule is preceded by a period of persistent headache, such headache being invariably temporal or occipital.
Robert Jones, in his paper, states that of the 133 cases who, from the nature of their work (painters, plumbers, etc.), were liable to lead impregnation, 19 had signs of poisoning upon admission, whilst 22 had a distinct history of lead poisoning at some time or other. He gives the following analysis of the mental condition:
| Mania | 37 |
| Melancholia | 33 |
| Dementia | 19 |
| Dementia with epilepsy | 10 |
| Dementia with general paralysis | 24 |
| ? General paralysis | 7 |
| Alcoholic mania | 3 |
| 133 |
Savage[19] is of opinion that lead will produce any of the symptoms of general paralysis of the insane, and may even be a contributory cause of the disease, but no statistics are available of the Wassermann reaction in these cases. Goodall[20] refers to the fact that nerve poisons, such as syphilis, alcohol, and fevers, injury or sunstroke, which are intermediate in fixity between alcohol and lead, seem to have an intermediate influence in the production of general paralysis.
Jones states that the mental symptoms found in the cases are to be grouped amongst one or other of the following varieties:
1. Of a toxæmic nature, with sensory disturbances, which tend to get well rapidly.
2. Hallucinations of sight and hearing, more chronic in nature, which may be permanent. The delusions in this class are almost invariably those of being poisoned or followed about, and are in the main persecutory.
3. Those resembling general paralysis with tremors, increased knee-jerk, inco-ordination, accompanied with listlessness amounting to profound dementia, but which tend to get well.
—Two main forms of eye change are to be found amongst lead-workers. In the first place, temporary and sudden amaurosis makes its appearance, due no doubt to vascular change,[159] either vaso-motor or hæmorrhagic. The trouble may occur in one or both eyes, may come on gradually, the patient merely being unable to distinguish letters or faces at a distance, or he may become suddenly totally blind. In the majority of cases the affection disappears under treatment, but in a small number of cases total blindness persists.
Occasionally nystagmus may be seen, but is not a common symptom; but dilatation of the pupils, quite apart from retinal changes, is not unusual. Inequality of the pupils may be observed, but partial dilatation of both pupils is more common, and is often associated with early anæmia. Conjunctival hæmorrhages are to be noted from time to time, without obvious cause, such as injury, etc., but in the majority of cases these have been associated with other symptoms.
The first feature noticed in the eye is loss of brightness, and a curious lack-lustre of the eyes of persons intoxicated by lead is one of the general features making up the saturnine cachexia. Loss of brightness of the eye is associated in many other diseases with anæmia, but is particularly prominent in lead poisoning, much more so than is to be accounted for alone by the degree of blood-destruction, and is a point of which the examining surgeon should always take notice.
One other form of eye change requires attention—namely, retinal changes due to circulatory disturbances. In an advanced case the whole picture is one of severe albuminuric retinitis, but in the earlier stages some engorgement of the vessels without alteration of the surrounding tissue is seen. Elschnig[21] associated this alteration in the vessels of the eye with vaso-motor changes caused by direct action of the poison, producing a vascular constriction or dilatation, and is inclined to regard the kidney disease frequently associated with this condition of the eye as something quite apart. He would regard the two affections as independent, and merely correlated through their common origin—lead intoxication. It has even been suggested by some observers that the change in the eye is secondary to cerebral œdema. Thus Mannaberg[22] regards the encephalitis of saturnine origin as associated with chronic œdema of the brain and spinal cord, which thus produces reflex irritation of the nervous system of the eye. Bikler[23] and Weber[24] consider the symptoms as circulatory. From whatever cause the disease is set up, sooner or later changes in the[160] form of obliterative arteritis take place, with gradual but ultimately complete loss of vision.
There are said to be no characteristic eye symptoms in acute cases of lead poisoning, whereas with chronic lead poisoning in many cases there is central and peripheral affection. The affections may be further divided into subjective and objective. Many of the subjective symptoms, such as loss of sight and blindness, are associated with definite eye lesions, which may be seen with the ophthalmoscope, but other definitely objective lesions may be present without any influence on sight to commence with. Folker[25] describes five cases of lead amblyopia in lead-workers from a pottery district, in all of which there was a peculiar symptom—the gradual failing of sight associated with colour flashes. When examined, the discs were described as white, and the vessels small.
Lockhart Gibson[26], in describing the cases of eye disease amongst the children in Queensland, found one symptom apparently in all the eyes examined—namely, great swelling of the discs. This swelling of the discs might be accompanied with no loss of sight whatever, and at other times had been accompanied with defective sight for many months previously. Some of the discs were excessively swollen. There were also to be seen patches of pigment and irregular swelling of the vessels, but no definite hæmorrhages. In the more acute cases, and particularly those associated with complete paralysis of the ocular muscles, total blindness usually followed.
As a rule, when complete amaurosis occurs in lead poisoning, blindness follows through double optic neuritis or neuro-retinitis, but amblyopia may be present without fundus changes. Occasionally the loss of sight may be regarded as of central origin. The renal disease so often associated with lead poisoning may cause the retinal changes accompanying it. An albuminuric neuro-retinitis may occur without albumin in the urine. As a rule, the eye of a lead-worker reacts to light and accommodation. Ophthalmoscopic examination may show very pink discs, patches of pigment scattered about irregularly outside the discs, with occasional definite hæmorrhage. The edge of the discs may show blurring, with further sclerosis and peri-arteritis of the vessels, a white sheath around the arteries being often visible. The neuritis on one or both sides may be associated with disturbances of sight, and diffusely red and cloudy papilla,[161] with swelling or hæmorrhages. In choroidal atrophy pigmentation may also be seen.
—One further point may be referred to in relation to the muscular system—namely, the occurrence of pain of a rheumatic type. Quite a number of cases of mild degrees of lead poisoning complain of arthralgic symptoms—that is to say, “rheumatism.” Careful examination of such cases shows no evidence that the pain is a true arthralgia, neither does it seem to have a true relation to gout. The pain as a rule is referable to the muscles themselves, and in such instances digital examination of the muscle in the region of the pain generally exhibits deep-seated tenderness. There does not appear to be any special marked tenderness along the trunks of the nerves supplying the muscle, nor is there evidence of hyperæsthesia of the skin. Such hyperæsthesia does occur in lead poisoning, but is generally associated with cerebral lesions. The pain, therefore, must be regarded rather as myalgic, and intercostal distribution of the pain is not infrequent; but although the symptom is one that is often complained of, it is an exceedingly difficult one to differentiate from other myalgias as a definite symptom of lead poisoning. The chief point in favour of the inclusion of this so-called lead rheumatism as a symptom of lead poisoning is the frequency with which it is noted in the reports by certifying surgeons. While, therefore, having no evidence to regard it as necessarily a definite symptom of poisoning, it is one which has been recorded in a considerable number of cases. As has been already suggested, there is some reason to think these myalgic pains may be due to minute hæmorrhages taking place in the muscles, thereby producing localized irritation to some extent comparable with the “bends” of divers.
—Very real difficulty exists in determining from naked-eye appearances at a post-mortem examination whether the cause of death be due to chronic plumbism or not. The changes produced by several other forms of intoxication, notably chronic alcoholism, produce many of the same changes in the tissues as lead. Inspection of the organs in the case of plumbism can only give rise to a surmise that the cause of death is due to lead poisoning.
There are, however, certain macroscopical appearances at an autopsy in the case of saturnism which should be carefully noted,[162] and although alone they do not constitute sufficient evidence upon which to pass a definite opinion, they are still important as diagnostic signs in the light of histological and chemical examination.
Particular note should be paid at an autopsy of a case of suspected lead poisoning to the following points:
1. Mouth, for the presence or absence of blue line, which, if present, must be examined with a lens.
2. General condition of the abdominal viscera, and particularly of the mesenteric and perinephritic fat. In plumbism this is invariably reduced in quantity.
3. The condition of the mesenteric vessels, as to whether or not they are engorged with blood, or whether or not leakage appears to have taken place.
4. General condition of the arteries, for the presence of atheroma, etc.
5. The heart muscle, which in plumbism is generally pale, flabby, and with a tendency to general dilatation of the cavities.
6. Intestines.
(a) The presence of injection of the muscular coat, particularly the lower portion of the intestine, and about the ileo-cæcal valve.
(b) The presence or absence of minute ulcerations, or even hæmorrhages along the intestine, even in the mucosa of the stomach.
(c) The presence of dark staining in the coats of the lower intestine, not altogether disappearing when washed under a gentle stream of water. Should there be any evidence of this staining, it is highly important to remove some of the fæces, as well as a portion of the intestine, for chemical examination.
7. The condition of the liver, which in poisoning by lead, as by alcohol, frequently shows a considerable amount of enlargement, and may even show patches of perihepatitis due to secondary causes. But the cirrhosis occurring in lead poisoning is not so great as in alcohol. In lead poisoning the liver as a rule is large and soft, and engorged with blood.
8. The kidney, for signs of interstitial rather than tubular nephritis, adherent capsule, and blood-stained exudate.
9. If paresis of any sort has been present during the illness, examination of the cord and brain should be made with especial[163] care, and in addition the nerves on the affected side supplying the affected muscles should also be examined. In the brain definite small but coarse hæmorrhages may be occasionally observed, but as a rule the only signs to be found are injection of the cortical vessels, frequently over certain definite areas, and not involving the whole of the vascular system of the brain. Minute hæmorrhages may be also found in the spinal cord.
For the purposes of histological examination, a portion of the following organs should be removed and placed in a 5 per cent. formalin solution at once: Liver, spleen, kidney, intestine, the last-named specimen being selected from any area which shows injection, or ulceration, or dark staining.
Smears may also be made from the bone-marrow, as in prolonged anæmia of saturnine origin definite changes may at times be found in the bone-marrow cells.
Where paresis has existed, a portion of the particular nerve supplying the muscles should be obtained, and histological examination made, as well as a portion of the cord above the lesion, and where cerebral symptoms have been present, a portion of the brain, the portions taken being part of that showing engorgement of the vessels. For the nervous tissue generally, it is better to place some of the specimens in Müller’s solution, and others in spirit. Equal parts of Müller’s solution and formalin may be used if desired.
—For the purposes of chemical examination, any of the organs which appear to be mainly affected by chronic inflammation may serve, but it is usually important to examine the brain, kidney, and liver. If any dark staining exist in the intestine, a portion of this, together with the contained fæces, should be removed. It is better to tie ligatures round the intestine, and divide the coat between the ligatures, and place the whole of the specimen in dilute formalin. Specimens thus obtained should be sent off for examination at once. The whole of the organ need not necessarily be despatched for examination in every case, but if only a portion is sent, it is essential that the weight of the whole organ be accurately taken before any portion is removed, and the total weight noted with the specimen when sent.
[164]
[1] Tanquerel: Traité des Maladies de Plomb ou Saturnines. Paris, 1839.
[2] Lancereaux: Gaz. Méd., 1862; Tribune Méd., 1896.
[3] Meillère, G.: Le Saturnisme, chap. iv.
[4] Teleky: Deutsch Zeitschrift für Nerven Heilk., vol. xxxvii., 1909.
[5] Déjerine-Klumpke: Thesis on Des Polynephrites en Général et des Paralysies et Atrophiques Saturnines en Particulier. Paris, 1889.
[6] Teleky: Ibid.
[7] Teleky: Ibid.
[8] Gombault: Arch. Phys., 1873.
[9] Moebius: Ueber einige Ungewöhnliche Fälle von Bleilähmung. Cent. für Nervenheilk. 1886.
[10] Tanquerel: Ibid.
[11] Sajous: Archiv für Laryng., iii., 1882.
[12] Morell Mackenzie: Brit. Med. Journ., epitome, p. 1202, 1893.
[13] Seifert: Berl. Klin. Woch., 1884.
[14] Lockhart Gibson: Brit. Med. Journ., vol. ii., p. 1488, 1908.
[15] Galezowski: Jahr. f. Aug., p. 382, 1877.
[16] Folker: Brit. Med. Journ., ii., p. 1556, 1898.
[17] Robert Jones: Brit. Med. Journ., September 22, 1900.
[18] Rayner: Journ. of Mental Science, 1880.
[19] Savage: Clifford Allbutt’s Medicine, vol. vii., p. 657.
[20] Goodall: Ibid., p. 693.
[21] Elschnig: Wien. Med. Woch., Nos. xxvii, xxix., 1898.
[22] Mannaberg: Ber. Klin. Woch., 1896.
[23] Bikler: Arch. für Augenheilk., b. xl., 1900.
[24] Weber: Thèse de Paris, 1884.
[25] Folker: Ibid.
[26] Lockhart Gibson: Ibid.
[165]
Very great assistance is afforded by chemical and histological diagnosis in the determination of cases of lead poisoning, especially when the case is likely to involve proceedings under the Workmen’s Compensation Act. In addition, a large amount of information is afforded to the certifying or appointed surgeon and the medical practitioner by adoption of certain easily carried out methods of diagnosis. It will be our purpose in the present chapter to describe as far as possible methods by which chemical or other investigation of a case of lead poisoning can be pursued, and the clinical methods of diagnosis which are applied in ordinary routine.
The majority of the methods described, especially the chemical investigation of material obtained from alleged fatal lead poisoning for the purpose of determining the presence or absence of lead, the histological examination of such tissues, and the examination quantitatively of excretions for lead, are processes which can only be carried out in a fully equipped laboratory, and certainly do not belong to the ordinary routine of medical work. The medical practitioner cannot be expected in the ordinary course of his routine work to examine blood-films for basophile staining or to make differential blood-counts. Especially is this the case in the routine examination of large numbers of factory hands. Further, many of the processes involved in either the chemical or histological examination of the tissues require so much special apparatus, without which such work cannot be undertaken, that the mere cost of the necessary instruments precludes the investigation being carried on except in special laboratories. At the same time, our purpose is to point out how additional methods of research may be made use of in obscure cases, and how recourse should be had to a well-equipped[166] laboratory in doubtful cases. Further, the coroner, when ordering a post-mortem examination, may ask for a histological and chemical examination.
—The presence of lead may require to be determined qualitatively and quantitatively, and the procedure may differ slightly as to which process it is necessary to adopt. The quantitative determination of the amount of lead present in organs or excretions of the body is of far more importance than the estimation or determination of the fact of its presence. We have already referred to the work of Gautier[1], who has found lead present in the tissue of normal persons with such constancy that French observers, at any rate, now speak of “normal lead,” to distinguish it from lead which may be found in pathological conditions. There is, however, little doubt that the quantity of lead existing in the human body is exceedingly small. It is possible, with certain refined methods of chemical examination, that qualitative traces of this substance might be found. On the other hand, the methods of determination of lead are some of the most difficult in toxicological analyses, on account of the presence of other metals, particularly iron, which are exceedingly difficult to get rid of, and may easily lead to errors.
—The group reagent for lead is sulphuretted hydrogen in acid or alkaline solution. Lead is precipitated by this reagent as a black precipitate. Where no other metals are present, and where no organic matter is present at the same time, there is very little difficulty, and the usual method of the determination of lead in water by means of sulphuretted hydrogen is exceedingly easy.
Potassium iodide gives a yellow precipitate soluble on warming, and forms large crystals on the tube. Hydrochloric acid and chlorides give needle-shaped crystals, soluble in heat, and crystallizing out when cold. But the double chloride of potassium and lead is more soluble in heat, and still more soluble in cold, than is the pure chloride. This fact is made use of in the process to be described presently in separating lead from organic mixtures.
The direct examination is rarely possible or satisfactory, but the potassium cupric acetate method is one that may be applied to the qualitative estimation of lead in the tissues.
[167]
—Dry and incinerate the material to be tested. Extract with hot dilute nitric acid, after repeated incinerations, finally extracting with ammonium acetate. Filter, incinerate, and take up the residue in dilute nitric acid. Evaporate to dryness and add a few drops of dilute acetic acid, and transfer drop to microscope slide.
To the drop on the microscope slide add one drop of dilute copper acetate solution, and two to three drops of saturated solution of potassium nitrate. Stir up the drops and well mix with a platinum wire, allow to stand for a few minutes, and then examine with a two-thirds objective. If lead is present, violet-black cubes of potassium copper lead nitrate appear (K2CuPb[NO3]).
This test is said to give a reaction in the presence of 0·00003 gramme.
—The quantity of lead passed by the kidneys into the urine is always small, even in acute lead poisoning. Further, the lead is excreted in organic combination, and is therefore difficult to detect. For absolute quantitative examination it is essential to evaporate the whole bulk, using at least ¹⁄₂ gallon of the fluid, and proceeding with the concentrate in the manner indicated in the estimation of lead.
For the qualitative examination many methods have been suggested, but all of them are more or less fallacious.
In certain instances in acute lead poisoning, or where a relatively large quantity of lead is excreted by the kidney, acidulation of the fluid with strong sulphuric acid direct will at times produce a precipitate of lead sulphate, which may be filtered off and the filtrate examined by the usual tests—namely:
A white precipitate with dilute sulphuric acid.
A yellow precipitate with potassium chromate, hardly soluble in nitric acid, but soluble in alkalies.
A blue flame on heating on a platinum wire, and finally, if sufficient substance is present, the reduction to metallic form with the blowpipe flame.
A method has been recommended of suspending a small bag of calcium sulphide in the sample to be examined, the supposition being that, if the calcium sulphide in the bag showed blackening, it would be due necessarily to lead. This is highly questionable,[168] and in the hands of one of us (K. W. G.) has not given satisfactory results.
A further method, which is quite simple in application, and occasionally gives confirmatory results, may be applied in the following manner: The urine to be examined is inoculated with the Bacillus coli communis. For this purpose a small quantity of fæces may be used. The B. coli in its growth makes use of the organic substances in the urine, and at the same time sets free sulphuretted hydrogen. The urine left is filtered, the filtrate dissolved in 10 per cent. nitric acid (minimal quantity), and the filtrate examined by the usual tests. This method has occasionally given quite good results in the hands of one of us (K. W. G.), and is, moreover, an exceedingly easy one to carry out.
Passing sulphuretted hydrogen direct through the fluid is of no value, as it is necessary first of all to split up the organic compound before it will react to sulphuretted hydrogen.
—Of all the methods at present in use for the estimation of the presence of lead in the urine, the electro-chemical gives by far the most satisfactory results. Several methods are described:
The first method consists in using magnesium, which is left for some hours in the urine, which has been previously strongly acidulated. In this manner Marsden and Abram[2] say that they have been able to detect 1 part in 50,000 in the urine without difficulty. The method adopted is as follows:
“A strip of pure magnesium is placed in the fluid to be examined. Ammonium oxalate in the proportion of about 1 gramme to 150 c.c. is added. If lead is present, it is deposited on the magnesium. A deposit is seen within half an hour, but we have usually left it twenty-four hours. The slip is then washed with distilled water and dried. Confirmatory tests: (1) Warm the slip with a crystal of iodine (yellow iodide proves lead, cadmium may be ignored); (2) dissolve deposit in HNO3, and apply usual tests. The magnesium can be used again after careful washing with acid and distilled water. The surface of the magnesium, when used, must be bright and free from oxide. The delicacy of the method has been tested with aqueous solutions containing known quantities of lead, also with normal urine to which known quantities of lead have been added. In all cases a control experiment was performed to insure the freedom of the materials[169] from lead. Lead has been detected when present in the proportion of 1 part to 50,000, whether in simple aqueous solution or in urine.”
Shufflebotham and Mellor[3] describe the following method as one by which lead may be detected in organic tissue, and in each case this necessitated a large amount of evaporation. The method has value, but the difficulty of dealing with large quantities of fuming nitric acid, and the addition of this acid from time to time during the operations, render it difficult unless a good fume chamber is at hand. Shufflebotham and Mellor state that they obtained no reaction with the potassium chloride-hydrochloric acid method suggested by Dixon Mann.
“On the Detection of Lead in Urine and Post-Mortem Specimens.—A piece of kidney of 20 c.c. capacity was cut up into about a dozen pieces. These were placed in an evaporating basin, and about 50 c.c. of fuming nitric acid were poured into the dish. Dense brown fumes of nitrogen oxides were evolved. When the action had subsided (in from two to three minutes), the dish was placed upon a sheet of asbestos, and allowed to simmer over the Bunsen flame for about an hour. If the frothing appears in danger of running over the sides of the dish, stirring with a glass rod or removal of the flame for a short time may be necessary. Twenty-five c.c. of the fuming acid were added at intervals of a quarter of an hour, and this process was repeated three times. The destruction of the organic matter was so complete that the whole of the piece of kidney passed into complete solution. The solution was then evaporated down to a few c.c., neutralized with caustic soda, filtered, and treated with hydrogen sulphide. A dark-brown precipitate of lead sulphide was obtained. With potassium chromate a yellow precipitate of lead chromate was obtained with the same specimen of kidney which gave a negative result with the KClO3-HCl method of destroying the organic matter. Our reagents, dishes, etc., were then examined with a blank test, but we found no lead.
“Urine.—We then sought the presence of lead in the urine of Cases 2, 3, and 4. Half a gallon of urine was evaporated down to dryness in each of two basins. In one basin the residue was heated until it was charred. Both residues were then treated separately with fuming nitric acid, as just described. The uncharred residue passed into solution, and on cooling deposited a white sediment. The mother-liquor was neutralized and tested in the usual way. A brown precipitate of lead sulphide was obtained in Case 2, while in Case 3 a well-marked black precipitate was obtained. The urine of Case 4 gave a negative result. The charred residue did not pass completely into solution, and the tests for lead were not so well defined as when the residue was uncharred. This shows that care must be taken to prevent charring of the residue during evaporation.”
A method has recently been described by Hebert[4]—a modification of Trillet’s. This method is based upon the fact that peroxide of lead, when mixed with tetramethyl of diphenyl-methylen, gives in acetic acid solution a fine blue coloration. Unfortunately, a number of other peroxides give the same blue coloration, amongst them manganese, potash, copper, magnesium. In addition, the sodium peroxide used to convert the lead present into the peroxide also gives a bright blue coloration with the reagent, even if present in minute quantities.
[170]
The test is made in the following manner: The substance is incinerated, sulphuric acid added in the usual way, and the substance evaporated to dryness. It is treated with a cold solution of sodium hypochlorite. The hypochlorite is then removed partly by washing and partly by heating, and the reagent is then added directly to the substance in the capsule, and if a peroxide is present the blue colour results.
Unfortunately, this dissociation-point of the hypochlorite and the temperature at which peroxide of lead is changed back again to the oxide are very close together, being only about 25° C. In addition, it is very difficult to remove the last traces of the substances which give a blue coloration in addition to lead. One of us (K. W. G.) has made extensive trials with this method, as, if it had been a reliable process, it would have been one which would have considerably facilitated the estimation of lead in small quantities. The method has been adopted by certain French observers, who by drawing 2 c.c. of blood from the median basilic vein, and estimating the lead present in this small amount by means of the blue coloration, have sought to show that at least 25 milligrammes of lead were circulating in the blood of the body. In addition to other grave considerations, the fact that the reagent itself is colourable by certain other peroxides which exist in the blood-ash renders these figures entirely untrustworthy.
—Two methods may be used in the detection of lead in organic substances, either in organic fluids or in solids, and are generally termed the “wet” and “dry” methods, from the original treatment of the substance.
In the dry method the substance is incinerated with or without the addition of sulphuric and nitric acid; in the wet the material is treated with hydrochloric acid and potassium chlorate. Subsequent treatment in both cases is on the same lines.
—Moist Method.—The substance which is suspected to contain the poison, if solid, is reduced to a pulp, and is mixed with sufficient water until of the consistency of thin gruel. The urine should be evaporated to one-fourth or one-sixth of its volume. Fæces should be well stirred up with distilled water. The substance is then placed in a large flask together with crystals of potassium chlorate; each 100 grammes of the substance require[171] 3 to 4 grammes of potassium chlorate. Pure HCl of the same weight as the original substance is then added, the flask is placed on a water-bath and gently heated. Care must be taken that the heating is not too brisk, as otherwise the evolution of the chlorine peroxide takes place too rapidly. If necessary, additional crystals of potassium chlorate are added from time to time until the fluid becomes limpid and of a slight yellow colour, or, if there is much organic matter, until it assumes the appearance and colour of thin oatmeal gruel. On account of more gradual evolution of chlorine, the chlorate that is present before the flask is heated acts much more energetically, weight for weight, than fragments added after the liquid is heated, as a great deal of the gas then escapes without rendering any service. If the substance contains sugar, starch, or alcohol, extra care must be taken to avoid frothing over. When the fluid contents are clear or reduced to a thin consistency, the liquid is transferred to an evaporating basin, and allowed to remain on a water-bath until the smell of chlorine has disappeared; it is then filtered while hot. The whole of the organic matter is not destroyed by this process, fatty substances especially being resistant; but if the organic matter is reduced to small fragments, any mineral poison present will be liberated.
The objections raised against this process are that some important poisons—such, for instance, as arsenic and antimony, specially the former—are liable to escape partially in the form of vapour, and that others, such as lead and silver, may remain as insoluble precipitates on the filter. As regards the first objection, it is to be observed that, when the hydrochloric acid is diluted with water (as in a moist method of destroying organic matter), any arsenic which may be present in the hot solution is not given off with its acid aqueous vapour, arsenious chloride dissolved in hydrochloric acid being volatile only when the solvent is concentrated. Any possibility of loss may be avoided, however, by furnishing the flask, in which the organic matter is being destroyed, with a condenser and receiver. The second objection, as far as lead is concerned, is met by taking care to filter the solution whilst hot; if only a limited amount of lead is present, it then remains in solution as chloride so long as the liquid is hot, and will consequently pass through the filter. A considerable quantity is kept in solution in the cold, as it forms a combination with potassium chloride, which is more soluble[172] than lead chloride alone. If a large amount is present, it will not all be found in the filtrate; the substance left on the filter, therefore, must always be tested for lead. In toxicological work, however, the amount of lead present is not as a rule more than will remain dissolved in the cold. Silver chloride, being insoluble either in hot or cold water, will not pass through the filter; consequently the salts of silver require dealing with in a special manner.
Dry Method.—This is effected by heating the finely divided substance to redness, so that it is either carbonized or completely incinerated. When cold, the residue is drenched with nitric acid, and sufficient heat is afterwards applied to drive off the free acid. The nitrate of the metal is then dissolved in water, filtered, and dealt with according to the kind of metal present.
The dry method is unsuitable in the case of the more volatile metals, as arsenic, antimony, and, in a lesser degree, lead, tin, and zinc. Further, it is extremely difficult and troublesome to carry out with large masses of organic matter. It is convenient with small amounts, and in the absence of the more volatile metals yields good results.
The following two methods are given by Glaister[6] on the one hand, and Dixon Mann[7] on the other. Both methods are good. It will be seen that Glaister recommends the estimation of the lead as sulphide.
When minute quantities of lead are present in combination with large amounts of organic matter, the dry process is tedious, difficult to carry out, and uncertain in its results. The plan adopted in the elimination of lead by Dixon Mann is as follows:
The urine is evaporated down to the consistency of gruel, and the fæces mixed in distilled water to a like consistency. They are then treated by the wet method, as given above. The filtrate after cooling is placed in a glass cell, the bottom of which consists of a sheet of vegetable parchment; the cell is immersed to such a depth in a deeper cell, containing distilled water acidulated with a few drops of sulphuric acid, that the liquids in the inner and outer cells stand at the same level. A piece of platinum-foil enclosing a surface of about 50 square centimetres, constituting the kathode, was submerged in the liquid contained in the inner cell, a similar piece of platinum-foil, constituting the anode, being immersed in the outer cell. The pieces of foil are so placed as to be opposite each other,[173] separated by the parchment diaphragm. A current, 3 or 4 volts, is then passed through for from six to eight hours, after which the foil is removed from the inner cell and gently washed and dried. The metallic lead is dissolved off the foil with dilute nitric acid aided by heat, and after driving off most of the free acid the solution is decomposed with dilute sulphuric acid with an equal volume of alcohol added. It is then set aside for twenty-four hours. The precipitate of lead sulphate is washed with water containing 12 per cent. of alcohol, until all the free acid is removed; it is then separated by decantation, ignited, and weighed. The amount of lead is calculated from the weight of the sulphate; 100 parts of sulphate are equal to 68·319 parts of metallic lead.
Whether the moist or the dry process is used, the residue after the primary filtration should be tested for lead, which may be present as sulphate and remain undissolved. If the original substance contains lead as sulphate, the salt should be dissolved with heat in an aqueous solution of ammonium tartrate to which a little free ammonia has been added, then precipitated with sulphuretted hydrogen (100 parts of lead sulphide equal 86·61 parts of metallic lead). It is better, however, to convert the sulphide into sulphate by treating it with nitric and subsequently sulphuric acid, after which it is ignited, weighed, and the amount of the metal calculated by the lead sulphate factor.
Having obtained the substance by decomposing the organic matter, two methods of estimation may be made use of:
(1) Colorimetric; (2) gravimetric.
Where the estimation is to be made gravimetrically, the substance is always obtained as a sulphate, and the lead estimated by weighing as a sulphate. This process is an exceedingly tedious one when a large number of small samples have to be estimated, as in the determination of the amount of lead dust present in the air. On the other hand, it is probable that the use of the balance in the estimation of lead as sulphate is more accurate where larger quantities up to 10 milligrammes are present; but where only 2 or 3 milligrammes are present in the amount of substance examined, the experimental error in washing is too large to warrant the expenditure of time required in this form of estimation, and the colorimetric method is used.
—Acidulate the organic substances, reduced to fine proportions, with nitric acid, heat[174] for some time, then permit to cool; filter, wash residue, and mix washings with filtrate; concentrate filtrate; pass H2S; place mixture in warm place to allow precipitate to settle; after which decant supernatant fluid, collect precipitate on tared filter, thoroughly wash, dry on water-bath, and weigh. One part of sulphide is equivalent to 0·9331 part of lead oxide and 1·5837 parts of acetate of lead.
The electrolytic method is better adapted for the detection of minute quantities of lead, as, for example, in the urine or fæces or in vomited matter. The urine may be evaporated to a viscous state; the others, finely broken up, are treated in the same way, after HCl is added, as recommended above, the mixture heated, and pinches of powdered chlorate of potash added, as necessary, to break down organic matter. The heating is continued until the odour of chlorine disappears, after which it is filtered and the filtrate allowed to cool. The filtrate is then placed in the outer cell of a two-celled arrangement, not unlike a dialyser, the bottom of which is formed of vegetable parchment, the outer cell containing distilled water acidulated with H2SO4. Into the inner cell is placed a piece of platinum-foil measuring about 50 square centimetres of exposed surface, which is connected with the kathode or negative pole of four Grove cells, and into the outer cell is placed a like-sized piece of platinum-foil connected with the anode or positive pole. These pieces of foil are so placed in relation to one another that they are only separated by the parchment. The galvanic circuit being now closed for some hours, any lead in the filtrate will be deposited on the platinum-foil connected with the kathode in the inner cell. The foil is then removed, carefully washed, and the metallic lead dissolved by dilute nitric acid aided by heat, after which the solution is concentrated until most of the free acid is driven off; dilute sulphuric acid is added to throw down the sulphate, alcohol being also added to expedite precipitation. The precipitate is allowed to settle for twenty-four to thirty-six hours, filtered on a tared filter, washed with water containing 12 per cent. of alcohol, dried, ignited, and weighed. One part of sulphate is equivalent to 0·68319 part of metallic lead and to 1·25 parts of acetate of lead.
The estimation of the lead, especially if the amount be small, may be more accurately made by the volumetric colorimetric[175] method. The metallic lead deposited on the platinum-foil is dissolved in nitric acid, and distilled water added, and an aliquot portion placed in a Nessler glass. A few drops of freshly-prepared H2S water, or H2S gas itself, may be added to or bubbled through the contents of the glass so as to form lead sulphide. The colour formed is now matched in a similar glass, using a standard solution of lead nitrate, and forming the lead sulphide as before.
Some have advocated the magnesium wire deposition test, originally devised by von Jaksch, and modified by Hill Abram, for the detection of lead in the urine of persons who are suspected to be suffering from chronic lead poisoning (see ante).
The colorimetric method of estimating lead has been made use of with very great success by Duckering in the estimation of lead in the air of potteries. The method is that devised by Mr. Vernon Harcourt[8], with a few modifications. The whole of the method is given, as observation on the quantity of lead present in the air of lead factories helps greatly in suggesting rational methods of precaution.
“After drying and weighing the filters, the following is the method. Solutions required:
“Nitric Acid.—One part of pure concentrated nitric acid to three parts of water.
“Caustic Soda.—One hundred grammes of pure caustic soda dissolved in 250 c.c. of water.
“Sugar.—A saturated solution of sugar in water.
“Sulphuretted Hydrogen.—A saturated solution of sulphuretted hydrogen in water.
“Coloured Solution.—Cotton-wool dissolved in concentrated nitric acid and evaporated to dryness, and the residue dissolved in a little water and filtered; the solution is deep yellow in colour.
“Standard Lead.—A solution of lead acetate or lead nitrate made up to contain exactly 0·0001 gramme of lead per c.c. of solution.
“The bulk of the dust in the filter was removed to a beaker (No. 1), by gently tapping the inverted funnel. The cotton-wool was then removed from the funnel, and the upper one-third, containing the remainder of the dust, was cut off and added to the dust in beaker No. 1. The remainder of the cotton-wool was placed in a second beaker (No. 2); 2¹⁄₂ c.c. of hot nitric acid was dropped on the dust in beaker No. 1 from a pipette, a little water added, and the whole heated. The solution was filtered into a 50 c.c. Nessler glass, and the liquid remaining in the cotton-wool also removed by squeezing the wool with a glass rod against the side of the beaker. The cotton-wool in beaker No. 2 was similarly extracted with 2 c.c. of nitric acid, and the solution added to that remaining in beaker No. 1. The liquid was heated, the cotton-wool macerated in it and filtered as before. The wool was then washed with hot water about three or four times, and the liquid filtered into the Nessler glass. A number of standards were then made up by running into Nessler glasses, from a burette, varying amounts of standard lead solution covering a fair range. Usually five standards were made up, containing 0·5, 0·8, 1·0, 1·2, and 1·4 c.c. lead solution, depending on the volume of the air aspirated and the quantity of lead expected in the known weight of dust found. To each standard 4¹⁄₂ c.c. nitric acid was added, and 5 c.c. of the caustic soda solution and 4 c.c. of the sugar solution were run into all the six solutions—i.e., one test and five standards—from pipettes. It was invariably found that the test was coloured faintly yellow, and if this is not allowed for in the standards high results are obtained. Hence a drop or two of the coloured solution (see solutions required) was added to the standards placed on white paper till they matched the test. Lastly, to[176] the contents of each of the six glasses was added 4 c.c. of sulphuretted hydrogen solution, and the liquid in each made up to the 50 c.c. mark, and the whole well stirred. Usually it was found that the colour of the test came somewhat deeper than that of one standard, and a drop or two of lead solution was added to the standard till its colour matched that of the test. The elaborate method of making up a number of standards was adopted because it was found that any other way gave high results. In the way described many trial experiments were made, and they were invariably correct within half a drop of the standard solution.”
The estimation of the quantity of lead present in organic fluids, with the disturbing influence of the presence of other metals, is a factor which always complicates the use of the sulphuretted hydrogen colorimetric estimation. It is almost impossible, when dealing with fæces, with blood, or, on the other hand, with artificial digests containing bread and milk, to eliminate the disturbing influence of iron. Further, if steps are taken to remove the iron and other metals, so much loss takes place in the manipulations necessary that the results arrived at are not satisfactory. Whenever dealing with organic matter, such as urine and fæces, it is best to make a blank test with a similar quantity of the substance under examination obtained from other sources, and to subtract the error found due to iron, when a rather closer approximation may be arrived at.
So far as can be estimated, the minimal quantity of lead required to produce poisoning is 0·005 gramme per kilogramme of body weight; but, on the other hand, persons who have swallowed much larger doses than this have exhibited no symptoms of poisoning. There is every reason to suppose that lead absorbed through the lung produces a maximum toxic effect, and, from the estimation of the quantity of lead found in the body after death, it is highly probable that exceedingly minute quantities of lead have, when absorbed over long periods, produced changes not only by their actual presence in the tissues, but also have set up degenerative changes which progress even after the elimination of the metal from its local position.
—In addition to the chemical examination of tissues from a person who has died of suspected lead poisoning, it is of the highest importance to make histological examinations, as the naked-eye appearance of post-mortem examination is frequently insufficient to give any clue to the cause of the poisoning. Moreover, in a large number of instances the necropsy may exhibit a number of signs of disease, such, for instance, as granular kidney, cirrhosis of the liver, and[177] so forth, which are associated with diseases other than lead poisoning, and, in the absence of any present or past evidence of definite hæmorrhages found associated with the other lesions already mentioned, an ordinary autopsy must be inconclusive. It is true that such pathological conditions are consistent with poisoning by lead; and if the individual has been a lead-worker, it is easy, but frequently erroneous, to conclude that the symptoms owe their origin to the worker’s occupation. We are entirely in sympathy with the remarks of King Alcock[9], who says:
“I plead none the less for an impartial investigation of the symptoms presented by a lead-worker, before assigning full or even partial responsibility of the disease to the occupation. If any and every departure from the normal in a lead-worker is at once assigned—the occupation being known—to plumbism, early diagnosis naturally presents very few difficulties to the exponents of such methods. And however severely we may condemn in the abstract such a careless, unscientific attitude, the tendency has, in practice, to be reckoned with and combated. The balance of probabilities would possibly suggest that the occupation is, after all, responsible, in one sense or another, for the more usual illnesses classically associated with the poison; nevertheless, the attending practitioner is in duty bound to take into consideration, and to estimate the relation of, all the concurrent causes of such symptoms.”
On the extremely unsatisfactory position the certifying surgeon may find himself in at a coroner’s inquest King Alcock says:
“The problem, from a strictly scientific point of view, is complicated—one might almost say that the truth is stifled—by the fact that the ætiological relations of the symptoms of any suspended worker are swamped by the insistent legal relations under which he claims and receives compensation.
“When once a formal certificate of suspension has been issued, which has embodied a recognition of lead as a cause of certain existing symptoms, then it becomes almost hopeless ever to reopen the question of causation of these or other supervening troubles, be their origin independent of lead or not. The doctor, in a legal cross-examination, is, in scientific honesty, bound to admit at last the bare possibility of any fantastic chain of remote sequelæ; and his protests against the probabilities of such[178] sequelæ are of no avail, as opposed to his own admission. The post, ergo propter, appeal carries the day easily.”
For the purpose of histological examination it is essential that portions of the brain, spinal cord, liver, kidney, and intestine, should be examined microscopically. The nervous tissue should be placed in formalin and Müller’s fluid, and a portion in alcohol for the examination of the fibres. The liver, kidney, etc., should be placed in 5 per cent. formaldehyde. The tissues are then treated by the ordinary histological methods, and sections prepared. With nervous tissue it is essential that those prepared for the examination of the cells should be made by the celloidin method; the others may be treated by imbedding in paraffin. The points to be sought for in the tissues are sufficiently indicated in the chapter on Pathology and Symptomatology, but may be briefly recapitulated:
In the brain, as well as in all the tissues, careful search should be made for minute microscopical hæmorrhages, and for evidences of old hæmorrhages in the form of small masses of fibrous tissue, etc. Parenchymatous degeneration, chromatolysis of nuclei, etc., nerve degeneration.
The arteries and veins should also receive close scrutiny, as the presence or absence of arteritis should be noted.
In the kidney particularly, search should be made for both interstitial and parenchymatous nephritis.
The liver frequently shows signs of microscopic hæmorrhage, and it is as well, in taking a portion of tissue for examination, to choose those portions which appear to be specially congested.
In the brain and spinal cord and nervous tissue, search is to be made for the same hæmorrhages as already noted. In addition, the condition of the nerve fibres should be noted, the presence or absence of periaxial neuritis, as well as degeneration of the axis cells, and the various ganglion cells both in the brain and spinal cord should be closely examined for chromatolysis and nuclear atrophy.
No evidence is afforded by micro-chemical tests of any of the sections thus obtained, except those of the lung. It may be possible in the case of the lung to determine the presence of lead granules in the alveolar cells, and attention should be paid to this. It is possible also that some evidence may be afforded by examination microscopically of the red bone-marrow.
[179]
The intestinal walls should be examined for evidence of lead particles.
If any dark staining, deep or superficial, be found in the intestine, it should be removed for chemical analysis. Necrotic areas of the intestinal wall should be sought for.
—For all practical purposes, the best stain for detection of basophile granules in the erythrocytes is Wright’s modification of Romanowski’s stain. This stain may be obtained in appropriate tablets, and may be prepared immediately before use, although a stain which has been standing for ten days or a fortnight gives much better results than a quite new stain. The stain consists of a solution of polychrome methylene blue, together with eosin in methyl alcohol, and the method of procedure is as follows:
Blood is obtained by a small puncture, and slides smeared and allowed to dry. Immediately on drying the slip is flooded with the stain, and allowed to remain for two minutes. This causes fixation. At the end of the two minutes the stain is diluted with an equal volume of distilled water, and allowed to remain on for a further three minutes. At the end of this time the stain is poured off, and the slip washed in distilled water for another three minutes, or until the characteristic purple-violet appearance is produced. It is better to examine such films with an oil-immersion lens, the oil being placed directly upon the films, and not covered with a cover-slip, as the action of Canada balsam tends to decolorize the blue. If such specimens are required to be kept, the oil may be washed off with xylol. It is possible to observe basophile staining with a good sixth, but an oil-immersion lens gives much the best result. The typical staining produced by this means gives darkish bodies scattered about the red corpuscles, staining sometimes deeply as the nuclei of the white corpuscles. In other cases the appearance is like that of fine dust scattered throughout the cell. In addition to these two forms, the whole red cell may take on a slight generalized lilac tint, the normal cells remaining free from granules, and stained red by the eosin. Search of 100 fields of the microscope should be made, and if no basophile granules are found in such fields it is unlikely that they will be found.
Basophile staining is not more pathognomonic of lead poisoning than of any other form of anæmia, but may be regarded as a highly important confirmatory diagnostic sign.
[180]
A differential count of the leucocytes present may be also made on the same film in which basophile staining is observed; 300 should be counted at least. In a typical case of lead poisoning it is found that diminution in the polymorphonuclear leucocytes has taken place with a corresponding increase of the lymphocytes, and possibly also the large mononuclears, and probably a slight increase in the number of eosinophiles.
This hæmatological method of diagnosis is of the utmost importance in lead poisoning. A differential count such as is given on p. 137, showing a large diminution in the polymorphonuclears, an increase in the lymphocytes, evidence of changes in the red cells, consisting of basophile staining, alteration in the shape of individual cells, poikilocytosis, with vacuolation, is strong presumptive evidence of lead absorption.
To complete the hæmatological examination, the hæmoglobin should be estimated. This is best performed with Haldane’s instrument—an exceedingly simple one to use. The estimation of the number of red cells and white cells present is useful, but does not by any means give such valuable information as does the differential count and search for basophile granules.
—Several methods are available for the estimation of the blood-pressure. The pressure may be roughly estimated as too high or too low by means of the finger. The presence of thickening of the arteries may be also estimated in this way, but for determining the absolute blood-pressure it is necessary to use one or other of the instruments on the market. The estimation of blood-pressure is an important point in relation to the suspected presence of arterio-sclerosis, and should be performed wherever possible. Sphygmographic tracings may also be taken. Such a tracing in a case of typical poisoning gives a peculiar form of curve, which, however, may be present in alcoholism and heavy work, and arterio-sclerosis of many types.
—In suspected cases of lead poisoning the examination of the urine may reveal the presence of lead. In addition, albumin is frequently present, especially in the early stages of kidney inflammation. The ordinary tests for albumin should be carried out, and it is also advisable to examine the urine spectroscopically, as at times hæmoglobin, methæmoglobin, hæmatoporphyrin, may be present in small quantities, each of which can be detected by means of spectroscopic examination.[181] Blood is not common in the urine of lead-poisoned persons, although microscopically hæmorrhages undoubtedly take place in the kidney. These hæmorrhages are interstitial, and as a rule do not cause any blood-pigment to be passed in a quantity that can be determined. It is as well, however, to centrifugalize the urine, and examine the deposit for red blood-cells.
The presence of hæmatoporphyrin, as suggested by Steinberg[10], is probably due to hæmorrhages in the intestine, and its presence in the urine should be regarded with suspicion in a lead-worker.
Where a lead-worker is suffering from continued absorption of lead, even without the manifestation of other symptoms, a change has been noted in the acidity of the blood—namely, a loss of normal alkalinity. The estimation of the alkalinity or acidity of the blood direct is an exceedingly difficult process, but much information may be obtained by careful estimation of the acidity of the urine, and of the acidity of the urine in relation particularly to the phosphates.
Joulie[11] has pointed out the extreme value which may be obtained from a knowledge of the urinary constituents by the means of estimation of the acidity with suchrate of chalk. The reagent is made by slaking lime in such a way that the resulting compound is practically dry. A quantity of this—about 25 grammes—is then thoroughly shaken up with 10 per cent. solution of cane-sugar, allowed to stand, and the solution titrated against decinormal acid until it is of one-twentieth normal. The urine is then estimated directly, the suchrate is run into the 25 c.c. of urine until a faint white flocculent precipitate appears. The number of c.c. of the solution of suchrate is then noted, and multiplied by the factor of the solution. This gives the acidity related to the phosphate and other organic acid contents, and is similar to the method used to determine the acidity of wines.
The second estimation consists of estimating the phosphates present by means of a standard solution of uranium nitrate, using either potassium ferrocyanide or cochineal as an indicator. The specific gravity of the urine is also determined. The result is then expressed in terms of this specific gravity, or, rather, in the terms of the density of the urine in relation to distilled water, and the whole answer returned per litre. By this method it is not necessary to obtain a twenty-four hours sample of the urine,[182] the urine passed first thing in the morning being taken for examination.
By using this density figure the quantity of acid and phosphate is expressed in relation to the density, the equation being—
The observed acidity The density per litre = Acidity per litre.
The phosphate content is expressed in the same manner, while the ratio of phosphate to acidity gives the ratio of excretion of phosphate to acidity.
There is in lead-workers a considerable diminution in the amount of phosphate excreted, and, as has been pointed out by Garrod and others, lead apparently produces alteration in the solubility of the uric acid content of the blood, and may therefore allow of its decomposition. Probably lead as a urate is stored up in the tissues. For further particulars of this method of the estimation of the urine, the reader is referred to “Urologie Pratique et Thérapeutique Nouvelle,” by H. Joulie.
An examination of the fæces of persons suspected of lead poisoning may often give definite results both of the presence of lead and hæmatoporphyrin. If small hæmorrhages have occurred high up in the intestine, the presence of hæmatoporphyrin in the fæces may result. The substance may be easily determined by means of the characteristic absorption bands. A quantity of fæces is taken and extracted with acid alcohol, and the filtrate examined spectroscopically. Urobilin bands are commonly present, and, particularly, where much constipation exists these bands are very well marked. There is no difficulty whatever, however, in distinguishing them from the characteristic bands of acid hæmatoporphyrin.
—The moist method or chemical examination given above is the best one to apply for the determination of lead in the fæces. As has already been pointed out, lead is commonly excreted in the fæces, and, if only about 2 milligrammes per diem are being excreted by the fæces in a lead-worker, the quantity cannot be regarded as indicative of poisoning. One of us (K. W. G.) has at times found as much as 8 to 10 milligrammes of lead excreted in the fæces of persons engaged in a lead factory, and exhibiting no signs or symptoms whatever of lead poisoning. If, however, the quantity[183] of lead in the fæces rises to anything above 6 milligrammes per diem, there is definite evidence of an increased absorption of lead, and if at the same time clinical symptoms be present, suggesting lead poisoning, such a chemical determination is of the first importance.
In estimating the presence of lead in fæces, it may be necessary to deal with the separation of iron, which may be precipitated as phosphate and filtered off, the quantitative estimation being proceeded with in the filtrate.
Lead is much more commonly present in the fæces of lead-workers than in the urine, and it is better to examine the fæces rather than the urine in suspected cases.
[1] Gautier: Meillère’s Le Saturnisme, p. 74.
[2] Marsden and Abram: The Lancet, vol. i., p. 164, 1897.
[3] Shufflebotham and Mellor: Ibid., vol. ii., p. 746, 1903.
[4] Hebert: Comptes Rendus, tome cxxxvi., p. 1205, 1903.
[5] Fresenius and von Babo: Liebig’s Annalen, vol. xlix., p. 287, 1884.
[6] Glaister: Medical Jurisprudence and Toxicology. 1910.
[7] Dixon Mann: Forensic Medicine and Toxicology, p. 496.
[8] Vernon Harcourt, A.: A Method for the Approximate Estimation of Small Quantities of Lead—Transactions of the Chemical Society, vol. cxvii., 1910.
[9] King Alcock, S.: Brit. Med. Journ., vol. i., p. 1371, June 24, 1905.
[10] Steinberg: International Congress Industrial Hygiene. Brussels, 1910.
[11] Joulie, H.: Urologie Pratique et Thérapeutique Nouvelle.
[184]
In laying down the general lines of treatment for both lead poisoning and lead absorption, it is essential in the first place to distinguish carefully between the two states; for although lead absorption may gradually drift into definite lead intoxication and lead poisoning, with all the classical symptoms associated with the saturnine cachexia, a large number of cases, particularly those in industrial processes, do not and should not progress beyond the early symptoms of lead absorption. The treatment, therefore, will depend in the first place on whether the case be one so constantly met with in industrial processes, where generalized symptoms of lead absorption are manifest without any definite and disabling symptoms traceable and sufficiently pronounced to enable a diagnosis of lead poisoning to be made.
The facts given in the chapter on Pathology, on the methods of entrance of lead, on the toxic manifestations, and the blood-changes, and, above all, the facts relating to microscopical hæmorrhages and other profound changes in the bloodvessels, point clearly to the lines along which the general treatment for amelioration, prevention, or cure of poisoning should be undertaken.
The treatment of the so-called “presaturnine state,” or what is preferably termed the “state of lead absorption,” is one that the appointed surgeon or certifying surgeon in lead factories or other processes in which lead is manufactured or used, is constantly called upon to treat. Lead poisoning is a definite entity as a disabling disease, whereas lead absorption, although the prodromal stage of such disease, cannot be defined as actual lead poisoning, as in many instances persons may show signs of continued lead absorption, but their powers of elimination can[185] be maintained at such a level that the ratio of absorption to elimination remains in equilibrium.
With the preventive treatment of lead poisoning we have dealt in another place (see p. 199). What is particularly required here is the medicinal treatment, which may be helpful in preventing lead absorption passing on to definite lead poisoning.
For many years it has been customary in the treatment of men employed in lead works to give occasional purgatives, and it is, moreover, a common and proper precaution to keep a stock of some simple aperient medicine, preferably saline composed of sodium sulphate and magnesium sulphate, at the works in charge of the foreman, so that any man who so desires may obtain a dose of an ordinary aperient mixture. We have seen from the pathological evidence that the largest proportion of lead is excreted by the bowel, and that, therefore, the sweeping away of the bowel contents—particularly where constipation is set up—will naturally tend to remove from the body a good deal of the lead which has been already excreted into the intestine and which may presumably become reabsorbed unless it be swept away. In a large electric accumulator factory Epsom salts in the form of the granular effervescing preparation is much appreciated. In winter 50 per cent., and in summer 90 per cent. of the men are said to take a daily dose. In an important white-lead works chocolate tablets containing hypo-(thio-)sulphite of sodium are supplied to the workers.
Another medicine made use of in lead works is the sulphuric acid lemonade, this being acidulated with sulphuric acid and flavoured with lemon. It is very questionable whether this substance has any definite effect in the special direction in which it is supposed to work—namely, that of forming an insoluble sulphate of lead in the stomach and so preventing its absorption. The use of this drug was suggested on the presumption that lead poisoning as a rule took its origin from the dust swallowed and converted into a soluble form in the stomach. As we have seen, there is very little evidence that this entrance of lead is of much importance, although it does occasionally take place. Furthermore, from the experiments of one of us [K. W. G.[1]], it has been found that the sulphate of lead is at any rate as soluble as other lead salts, such as white lead or litharge, when acted upon by normal gastric juice.
With regard to the drinks supplied to workers in lead factories,[186] it is highly important that some form of fluid should be supplied which the men may drink without harm, particularly in the more laborious forms of employment, and, above all, in the factories where smelting, desilverizing, etc., of lead is carried on. In these factories the use of some type of lemonade containing sodium citrate is to be recommended, as it has been shown that one of the pathological effects of lead absorption is to produce an increased viscosity of the blood, and the use of such drugs tends to some extent to diminish this. A drink containing a few grains of sodium citrate to the ounce and flavoured with lemon is freely drunk by workmen engaged in the laborious processes.
Finally, as a general routine treatment, it is advisable to keep at the factory some form of mixture containing iron, which may be given to those persons who are showing signs of slight anæmia, generally associated with some degree of constipation, and it is therefore better to use a form of iron cathartic. This medicine should also be kept in the care of the foreman, who will see that it is administered to the men properly. In this way any persons who at the weekly examination exhibit signs of anæmia may be promptly treated, and what is more, the surgeon is assured that the workmen in question actually obtain the medicine prescribed regularly.
During the routine weekly or monthly examination, or at whatever intervals the medical examination takes place, particular attention should be paid to the records kept of the state of health of the various persons, and whenever possible alteration of employment should always be enjoined when early signs of anæmia make their appearance.
The surgeon should spare no pains to determine if any of the workmen are confirmed alcoholics, and such persons should be removed from work in dangerous processes, while at the same time care should also be taken to eliminate any persons suffering from those diseases which are known to be predisposing causes of lead poisoning. The card system of registration of any symptoms noted or treatment given facilitates supervision of the health of the men.
In times of stress where some particularly dangerous process is in operation, as, for instance, where portions of a building which has become thoroughly impregnated with lead dust is being pulled down, or where machines are being altered, removed, or rebuilt, especial care should be exercised with the workmen[187] so employed, and it is advisable in such cases to adopt preventive measures on the supposition—generally correct—that such persons are absorbing a larger quantity of lead owing to their peculiarly dusty employment than they were under normal circumstances. At such times, also, it may be advisable to administer some form of mild iron cathartic to all persons employed in the factory for, say, a week at a time. It must not be supposed, however, that these methods of treatment in any way supersede the precautions for the prevention of lead poisoning by mechanical and hygienic means; they are merely additional precautions which may be put in force under special circumstances.
—The treatment of definite lead poisoning, as the treatment of lead absorption, is directed towards the elimination of the poison, the promotion of repair to the damaged tissues, and special treatment directed towards those special organs which suffer mostly in lead poisoning. At the same time, special treatment of urgent symptoms may be called for; but in the treatment of the urgent symptoms the fact of the general elimination of the poison must not be lost sight of.
We have already seen that the channel through which the poison leaves the body is mainly the fæces. Treatment must therefore be directed, as in the former instance (lead absorption), towards eliminating the poison by this means as much as possible, both by the use of enemata, and later the use of sulphate of magnesia, which may be added to the ordinary fluid enema; and it is far better in obstinate cases of constipation and colic to give enemata than to continue with the huge doses of salines or other aperients, such as croton-oil, elaterinum, or castor-oil.
—Lead colic may be simple, acute, recurrent, or chronic and continued. In whatever form colic appears pain is invariably referred to the lower part of the abdomen, frequently into the groins, and occasionally to the umbilicus. The pain has to be distinguished particularly from acute gastritis, and occasionally from appendicitis, and sometimes from that of typhoid fever. Acute colitis—not common in this country—and dysentery, may, to some extent, simulate the pain of lead colic, but John Hunter’s[2] original definition of “dry bellyache” conveys very vividly the type of pain. Occasionally diarrhœa may be met with, but as a rule obstinate constipation is present.[188] In continued colic, or chronic colic, sometimes lasting for several months, obstinate constipation is the rule. In the simple acute colic the pain passes off in the course of five or six days, generally disappearing about four days after the lower intestine has been thoroughly cleared.
The pain of lead colic is relieved by pressure upon the abdomen, whereas that of gastritis and most other forms of abdominal pain may be generally elicited along the descending colon and splenic flexure; mucus is commonly found in the stools, especially the first evacuation, after obstinate constipation occasionally of several days’ duration associated with an ordinary attack of lead colic. Blood may be passed, but this symptom is not common. The pain in the acute form is paroxysmal; it is rarely persistent, being typically intermittent. During the paroxysm distinct slowing of the pulse-rate with an increased blood-pressure takes place, and the administration of vaso-dilators—such, for instance, as amyl nitrite—during a paroxysm rapidly relieves the pain and lowers the blood-pressure, and in this way distinguishes acute colic of lead poisoning from, say, subacute appendicitis.
Vomiting may or may not be present, though the patient usually complains of feeling sick, but there may be at times vomiting of a frothy mucus.
It is unusual for a patient to die from acute colic, but acute paroxysms have been recorded in which yielding of the blood-vessels of the brain has occurred.
Recurrent colic is as a rule less severe than the simple acute form, but may last for several weeks, clearing up for three or four days at a time and then recurring with little diminution in violence from the first attack. Such cases are probably due to the gradual excretion of lead by the intestine, and should be treated on this supposition.
In the continued or chronic colic the pain may persist for as long as two months, during the whole of which time the patient complains of uneasiness and even constant pain in the lower part of the abdomen, which becomes considerably worse after each evacuation, and almost invariably is associated with exceedingly obstinate constipation. It is this type of case that olive-oil or liquid paraffin relieves, while in the acuter forms drastic purgatives such as castor-oil, croton-oil, or pulv. jalapæ comp. may be administered.
[189]
For the treatment of pain in colic one of the various vaso-dilators should be used, as, in addition to the spasm of the intestine, a very considerable vaso-constriction of the whole of the vessels in the mesenteric area occurs. Amyl nitrite gives immediate relief, but the effect passes off somewhat rapidly, whilst scopolamine, although taking somewhat longer to act, is better for continuous use, as its action is longer maintained. Sodium nitrite, liquor trinitrini, and antipyrin are also of use. Atropin may be used, but it is perhaps better given in conjunction with magnesium sulphate.
Whatever form of purgative is given, some form of anodyne should be combined. Drissole and Tanquerel[3] are said to have obtained excellent results with croton-oil; one drop is given, followed seven or eight hours later by another, and then by an enema of 2 pints of normal saline. After two or three days the croton-oil may be again given, one drop at a time each day. In addition, Tanquerel made use of belladonna and opium together, finding that their combined action was better than that of opium alone, as the physiological effect of belladonna probably assists in preventing the intestinal cramp.
Hoffmann[4] recommends the use of olive-oil and opium, giving 3 to 4 ounces of olive-oil. He says that this relieves the spasm of the pylorus, and is of particular use where severe vomiting is associated with the colic. This use of olive-oil, first suggested by Hoffmann in 1760, and revived by Weill and Duplant[5] in 1902, is somewhat interesting, in view of the modern tendency to administer paraffinum liquidum in the treatment of chronic constipation.
Briquet[6] recommends 4 grammes of alum and 4 grammes of dilute sulphuric acid three times daily, with the addition of 0·05 gramme of pulv. opii at night. Briquet says that although the purgative method rapidly diminishes the colic, the elimination of the poison does not take place as rapidly as by means of the treatment he recommends, though it is open to doubt whether the use of either of these two drugs is likely to produce any further neutralization or excretion of absorbed lead than sulphate of magnesia. It is quite certain that the magnesium sulphate does not act as a neutralizer of the poison, as in a factory where sulphate of lead is manufactured some cases of definite lead poisoning occurred, in which at least half must have been due to the inhalation of lead sulphate dust. Under[190] these circumstances it seems hardly worth while to attempt to form a sulphate of lead in the body. The action of magnesium sulphate and other salines, however, in promoting the flow of fluid towards the intestines, and rapidly diluting and washing out the contents, tend to eliminate such lead as has already been excreted into the bowel.
A number of other drugs have been given from time to time for the purpose of forming an insoluble compound with the metal in the intestine, such, for instance, as sulphur in many forms, which is still much used in French hospitals. Peyrow[7] advises sulphide of soda, whilst Meillère prefers potassium sulphide as being less irritating. He considers sulphuretted hydrogen a proper prophylactic against reabsorption. Both experimental work and clinical observation show that a change to sulphide does take place in the lower bowel, and that staining of this part of the intestine is due to lead sulphide; but as the figure on Plate II. shows, the lead may exist in the form of granules of a dark nature, deeply embedded in the intestinal wall, besides being situated in the exterior.
Stevens[8] suggests the use of ¹⁄₂-grain doses of calcium permanganate thrice daily to relieve pain.
A certain number of other drugs may be also made use of from the point of view of diminishing the pain, and one French observer advocates the hypodermic injection of cocaine, but it is doubtful whether any good would follow from such a procedure. Hypodermic injections of morphia should be given whenever the pain is great, and diaphoretics as well as diuretics should also be given, such, for instance, as ammonium acetate, citrate of potash, or soda. Chloroform water and chloral and bromine water may be also used, and when no other drug is at hand, the inhalation of chloroform will rapidly relieve the acute vaso-motor spasms associated with colic.
During the attack of colic, and for at least a day subsequent to its disappearance, the patient should be kept on a fluid diet; milk is best, and 10 grains of sodium citrate should be added to each glass of milk. After the colic has subsided, a light farinaceous diet should be given, and it is better not to give meat until at least a week has elapsed. Alcohol is to be avoided.
—As has been pointed out in Chapter VIII (p. 135), the anæmia of lead poisoning is one due to the destruction of the red blood-cells. This is evidenced not[191] only by the curious sallow complexion, by the occasional presence of hæmatoporphyrin in the fæces and urine, and often by the curious yellow of the sclerotics, but also by an increase in the viscosity of the blood itself. Moreover, the urine of persons suffering from lead poisoning is invariably highly coloured, and may even show the presence of methæmoglobin. As the anæmia is generally a symptom of continued lead absorption for a long period, and does not necessarily occur with every case of colic—in fact, acute colic may often supervene without any symptoms of continued anæmia—the persons suffering from lead anæmia should be removed from their direct contact with the dangerous processes, and should be given, if possible, work in the open air. Iron and arsenic may be used, preferably in combination, whilst the iodide of iron often gives good results. Whatever preparation of iron is given, care should always be exercised in avoiding any constipating effect, and the free action of the bowel should be maintained, together with a liberal supply of milk. Potassium iodide may be also given.
With regard to the action of potassium iodide, there is division of opinion amongst various physicians as to the efficacy of the drug in the elimination of lead from the body. At the same time a very large number of persons hold that the administration of fairly large doses of potassium iodide in the case of a person suffering from chronic lead absorption may at times be associated with sudden exacerbation of the disease, and that the drug apparently may determine the production of acute symptoms, such as encephalopathy or paralysis, when these have not been previous features of the case. Our experience supports this statement, and on more than one occasion one of us (K. W. G.) has seen a distinct increase of symptoms follow the administration of large doses of potassium iodide. From a comparison with other cases it seems that these symptoms would have been unlikely to make their appearance without some secondary cause. Against this point of view must be quoted further experiments already referred to by Zinn[9], who found that when lead iodide was administered to experimental animals iodine alone was found in the urine; but it must be pointed out that no estimations were made of the fæces, and it is possible that a certain amount of lead was eliminated in this way. What exactly is the action of iodide on the solubility of lead in the body it is difficult to say; yet[192] the use of iodine compounds has been followed with considerable success in a number of chronic inflammatory diseases, and it is possible that it may have the action of splitting off the particular lead compound from its organic association with the tissues, especially as it is well known that iodine plays a very important rôle in the process of cell metabolism. Another point which tends to support the use of iodine is the fact that the other two halogens, bromide and chloride, both of which enter largely into cell metabolism, also have a slightly beneficial effect on the excretion of lead. The dose of the iodine given should not be large to commence with, 3 grains three times a day is sufficient, the dose being run up to some 30 or 40 grains per diem, the symptoms meanwhile being carefully watched.
Other symptoms often associated with the anæmia of lead poisoning are—
—These pains are suggestive of muscular affection, and are possibly due to minute hæmorrhages occurring in the muscle tissue, which have been discovered in the muscles of experimentally poisoned animals. For the rheumatic pains diaphoretics and citrates of soda and potassium may be given.
—The lumbago constantly complained of in chronic lead poisoning and even in the early stage of lead absorption, is very generally related to chronic constipation rather than to a definite affection of the lumbo-sacral joints.
—Affections of the kidney associated with lead poisoning are almost entirely confined to sclerosis. The presence of albumin in the urine is not a very common symptom. As has been pointed out already, the presence of lead in the urine is by no means a regular feature of lead poisoning, though it may at times be present, and the urine should always be examined for changes in the kidneys; but as a number of cases of chronic lead poisoning are associated with alcohol poisoning, the changes in the kidney cell are almost certain to be present. On p. 95 the illustration showing the disease in the kidney produced by experimental dosage with lead, and the kidney of a fatal case of lead poisoning in a man who at the same time had a strong alcoholic history, shows fairly definitely the difference between these two points.
Acute nephritis occurs so rarely in the course of industrial lead poisoning that it cannot be considered to be a disease due to lead.
[193]
In chronic nephritis treatment should be along the ordinary lines and the same remark applies to enlargement of the liver.
—Symptoms due directly to disease of the heart are rarely caused by lead alone. The heart muscle may suffer in the same way as the other muscles of the body, and in lead poisoning in animals distinct hæmorrhages are found between the muscular fibres in the heart muscle, and it is therefore probable that a form of myocarditis may exist in lead poisoning. This, together with the increased arterial tension, may cause dilatation, but the symptoms are those related more to the general condition of arterio-sclerosis than to any direct heart lesion, and as a rule these symptoms do not call for any special treatment.
—With one or two exceptions, the diseases of the nervous system associated with lead intoxication only appear when actual lead poisoning is established. Certain evidences of affection of the nervous system are occasionally seen in the prodromal stage, or stage of lead absorption. These may be merely temporary and disappear often under treatment, by change of employment and reduction in the quantity of lead absorbed. Thus, dilatation of the pupils—the reaction to light being extremely sluggish or absent—is often a feature of the later stages of the condition of lead absorption. Tremor may also be a symptom, the outstretched hands exhibiting a fine undulatory movement, often increased on attempting to perform some act such as touching the nose, or touching the two fingers together, and when these symptoms occur they must always be regarded as of somewhat grave import. But it must be remembered that tremor may occur as a common complication of alcoholic cases, and further, follows excessively hard manual work, though there is usually little difficulty in distinguishing between the various forms.
The symptomology of nervous diseases associated with lead poisoning has already been carefully set out in Chapter IX., and the pathological changes underlying these symptoms in Chapter V.
Of the general treatment, little needs to be added to what has already been stated for the treatment of lead anæmia and general lead intoxication. Iron and arsenic (not strychnine, especially in presence of colic), and other similar drugs, should[194] be employed together with iodides either as potassium iodide or as an injection in the form of an organic compound, of which there are several varieties on the market.
The injection of normal serum has been advised, as well as saline injections, and in some instances venesection has been practised, but it is doubtful whether anything is to be gained by this form of treatment.
Further, it has been stated that some lead is excreted through the skin, and for this reason sulphur baths, bathing in sulphuretted hydrogen water, etc., have been recommended to neutralize any lead that has gained access to the skin. Serafini[10] has claimed that by means of electrolytic baths a certain amount of lead can be found present in the water after continuous passing of a current, and it has been supposed by these observers that the lead has been actually driven out of the body under the action of the electric current. It is, of course, possible that such lead as is discoverable in the water was merely that which had already become incorporated with the patient’s skin through mechanical contact.
Whatever form of treatment be adopted of a general type, the patient must certainly be removed from the chance of any further lead absorption; a person who is suffering from wrist-drop or other form of paresis should not be employed in any portion of a lead works where he may come into contact with any form of lead or its compounds for at least a year after the paresis has disappeared, and even then it is inadvisable for such a person to return to any form of dangerous lead work.
The electrical treatment of the injured nerves and muscles should be undertaken energetically; both the galvanic or faradic currents may be used. Probably the best form is the galvanic. A small medicinal battery may be utilized, the method of application being as follows: One pole of the battery should be placed over the affected muscle, and the other pole placed in a basin of water into which the patient’s hand is dipped. The current should then be passed. It is better not to use a current of too great intensity, particularly at the start, although it is found in practice that a much greater current can be borne in the early stages of the treatment than when the muscles and nerves commence to recover. As a rule the patient experiences no inconvenience whatever from a considerable current during the first week of his affection, but at the end of a fortnight or three[195] weeks less than one-third of the initial current can be borne. The current should not be passed continuously, but should be used for a short time and then shut off, being again switched on for five or six minutes, and then again shut off. The applications may also be modified by placing one hand in the vessel of water and stroking the affected muscle and nerve with the free electrode. The application of the current should be for not more than half an hour at a time, and may be applied twice in the twenty-four hours. It is quite easy to instruct the patient to perform the electrical treatment for himself in this manner when the paresis is affecting either the upper or lower extremity.
With the faradic current the circuit should be closed while the current is at a minimum, and then the quantity of current raised to some 15 to 20 milliampères.
For affections of the lower extremity the application may be made by means of one of the usual baths in which the foot is immersed, the other electrode being placed on the back or other suitable position. If both the lower extremities are involved, then both feet should be placed in a bath into each of which the source of electricity is connected.
Ionization by means of the faradic current may also be made use of. For this purpose one of the halogens, preferably iodine or chlorine, should be used, it being remembered that chlorine and iodine ions enter from the negative pole, so that in such a case the bath in which the affected limb is placed must be connected with the negative pole of the battery.
Subsequently, with either form of electrical treatment, the part should be well rubbed, and passive movements as well as massage are an advantage in promoting the return of normal function. As the muscles gradually return towards their normal state, graduated muscular exercises should be used.
When treated in the first week or two of the onset, lead paresis frequently recovers, and in a person suffering from lead palsy for the first time, confined only to the hands or to a group of muscles in the shoulder, prognosis is good. The prognosis of palsy of the lower limbs is not so good.
Paralysis of the facial nerve is occasionally seen in lead poisoning, and where this occurs it should be treated as previously recommended, by means of iodides in association with localized electrical treatment. One pole of the battery should be placed[196] below the external auditory meatus, and the other one passed over the face on the affected side.
In long-standing cases where no attempt has been made at treatment in the early stages of the disease, and where considerable muscle degeneration has already taken place, the prognosis as a rule is very bad. Efforts should always be made in an early case by passive movements and massage of the affected muscles to improve their nutrition as far as possible. The diet should be light, and alcohol should not be given at any time.
—The typical form of affection of the central cerebral nervous system caused by lead, is lead encephalopathy. The disease may be insidious in its onset, and may be preceded by a long stage of chronic headache with slight or total remissions. Headaches may last for several months before the actual acute stage of the disease is reached. In the examination of several brains of persons who have died from lead encephalitis, microscopic sections of the brain have shown signs of hæmorrhages which must have taken place some considerable time prior to death, and were no doubt associated with the headache that had been complained of for some time previously, before the onset of the fatal illness. (See Plate III.) Persistent headache occurring in a lead-worker should always be regarded with grave suspicion, and such a case should be treated on the assumption that it is an early case of lead encephalitis. Bromides and iodides should be given, and the patient placed in quiet surroundings, and fed on light, nutritious diet, and every attempt made to produce elimination of the poison.
In the acute attacks vaso-motor spasm is no doubt partially accountable for the symptoms, and various dilators, previously noted in discussing colic, may be made use of, such, for instance, as amyl nitrite, scopolamine, etc., whilst pyramidon, antipyrin, phenacetin, and other similar drugs may be given between the attacks. Under no circumstances should any person who has suffered from encephalitis or other cerebral symptom of lead poisoning be allowed to resume work in a lead industry.
The treatment of eye affections in lead poisoning requires little comment, as the essential treatment must be the same as in other cases, mainly devoted towards the elimination of the poison. Attempts may be made to treat paresis of the ocular muscles by means of mild electric currents, but of this[197] we have had no experience. About 50 per cent. of cases of lead amaurosis and amblyopia recover, but a number progress to total and permanent blindness, and prognosis in such cases must always be guarded.
—The prognosis of the first attacks of lead poisoning of simple colic or even slight unilateral paresis is good; practically all cases recover under proper treatment. It is unusual for a person to succumb to a first attack of simple colic, or paresis.
In most cases the serious forms of poisoning only make their appearance after three or four previous attacks of colic, but a single attack of paresis is much more frequently followed by a severe form of poisoning, such as encephalitis.
A limited number of persons are highly susceptible to lead poisoning, and these persons rapidly show their susceptibility when working in a dangerous lead process. Lead poisoning occurring in an alcoholic subject is more likely to result in paretic and mental symptoms than in a person who is not addicted to alcohol, and the prognosis of lead poisoning in an alcoholic is much less favourable than in the case of a normal person.
Mental symptoms very rarely follow from a single attack of lead colic, and as a rule do not become established under three or four attacks at least.
A small number of persons exposed to excessive doses of lead absorption through the lungs develop mental symptoms, such as acute encephalitis, without any prodromal stage. The prognosis in such cases is always exceedingly grave.
Sudden generalized forms of paralysis are not common in the early stages, but are invariably of grave import. A few cases of paresis, particularly those of the peroneal type, and affecting the lower limbs, become progressive, and eventually develop into a condition resembling progressive muscular atrophy with spinal cord degeneration.
The prognosis of simple colic in women is about as good as for males, but if an attack of abortion is associated with lead poisoning, eclampsia often supervenes and permanent mental derangement may follow. In the dementia associated with lead poisoning the prognosis is not so grave as in other forms of dementia, especially alcoholic, but depression is an unfavourable symptom. The mania of lead poisoning is not so noisy as that of alcoholic mania, but where there is suspicion of alcoholic as well as lead poisoning the prognosis is exceedingly grave.
[198]
As a rule the prognosis of cases of lead poisoning occurring in industrial conditions is more favourable when colic is a marked feature than when it is absent, and there is no doubt that the prognosis in cases of industrial lead poisoning at the present time is more favourable than it was before the introduction of exhaust ventilation and general medical supervision—a fact no doubt to be explained by the relative decrease in the amount of lead absorbed.
[1] Goadby, K. W.: Journ. of Hygiene, vol. ix., 1909.
[2] Hunter, John: Observations of Diseases of the Army in Jamaica. London, 1788.
[3] Drissole and Tanquerel: Meillère’s Le Saturnisme, p. 164.
[4] Hoffmann: Journ. de Méd., October, 1750.
[5] Weill and Duplant: Gazette des Hôpitaux, lxxix., 796, 1902.
[6] Briquet: Bull. Thérap., Août, 1857.
[7] Peyrow: Thèse de Paris, 1891.
[8] Stevens: Bulletin of Bureau of Labour, U.S.A., No. 95, p. 138, 1911.
[9] Zinn: Berl. Klin. Woch., Nr. 50, 1899.
[10] Serafini: Le Morgagni, No. 11, 1884.
[199]
—Lead fuses at 325° C. and boils at between 1450° and 1,600° C. It is volatile when heated to a cherry-red colour—about 550° C.
Experiments[A] carried out in the laboratory of a lead smelting works in London to determine the temperature at which leady fumes rise from the surface of open baths of molten lead, showed that unless pure lead is heated to about 500° C., and at the same time stirred, no appreciable fume comes off, and that from lead, at the same temperature, under ordinary working conditions, little or no lead in the form of oxide passes into the air. From lead that has been unrefined or which contains zinc—that is, lead in the earlier stages of its manufacture (in the reverberatory furnace)—leady fume was not given off at temperatures less than 760° C. even when stirred, because at a temperature of 600° C. the surface of the molten metal became covered with fluid slag, which will not allow any oxide to be given off. Impurities such as tin or antimony prevent the oxidation of molten lead at lower temperatures, and give it a bright, shiny colour. When heated to about 600° C., these impurities form a slag on the surface of the lead containing antimoniates and stannates of lead, which do not evolve lead fumes unless heated to temperatures never likely to be reached in open lead pots.[200] The reason why molten refined lead can give off lead fume more readily than those named is because the oxide formed on the surface is a dry powder and not in the form of slag. Hence, when the bath is stirred, some of the dry oxide is broken up and may rise into the air. When a bath of molten lead is not stirred at all, it can be heated to over 740° C. without finding oxide in the air aspirated—a temperature not obtained under ordinary working conditions.
[A] In these experiments air was aspirated through an iron funnel having an area of 113 square inches (12 inches diameter), placed at a height of 1¹⁄₂ inches above the molten metal, and connected to an iron tube 3 feet in length and ¹⁄₂ inch in diameter. Inside the iron tube was a glass tube, one end reaching own to the top of the funnel and the other connected with a tube containing pure loose asbestos wool, and continued down to a tightly stoppered bottle holding dilute sulphuric acid. Another glass tube connected this bottle with an aspirator. The asbestos tube was weighed before and after each test, and the asbestos then treated with nitric acid, and the lead determined volumetrically. In none of the tests made was lead found in the bottle containing sulphuric acid.
Were there nothing else to consider but escape of lead fume from a pot or bath of molten metal, obviously hooding over of the bath and removal of the fume from the atmosphere of the workroom would be unnecessary until this temperature was reached. Usually, however, the bath is kept standing exposed to the air, and the oxide which forms on the surface has to be skimmed off periodically, and whenever the ladle is emptied a small cloud of dust arises. Or at times, in certain processes, chemical interaction takes place in the bath, as in the dipping of hollow-ware articles previously cleaned in hydrochloric acid, with evolution of fume of volatile chloride of lead. Any vessel, therefore, of molten metallic lead in which skimming is necessary, or in which chemical action gives rise to fume, requires a hood and exhaust shaft, even although the temperature is little, if at all, above the melting-point—unless, indeed, a separate exhaust can be arranged for the removal of the dust immediately above the point where the skimmings are deposited.
Of many samples of dust collected in workrooms where there are baths of molten lead, it is impossible to say definitely how much of the lead present is due to fume, and how much to dust. Thus, a person tempering the tangs of files was attacked by plumbism, and a sample of dust collected from an electric pendent directly over the pot, at a height of 4 feet from the ground, was found to contain 15·6 per cent. of metallic lead. Similarly, a sample taken above a bath for tempering railway springs contained 48·1 per cent. metallic lead[1]. And, again, a sample collected from the top of the magazine of a linotype machine contained 8·18 per cent. Such analyses point to the necessity of enclosing, as far as possible, the sources of danger—either the fume or the dust, or both. Determination of the melting-point of the molten mass will often help in deciding whether there is risk of fume from the pot, and, if there is not (as in the sample of dust from the linotype machine referred to), will direct attention to the sources of dust[201] in the room. Proceeding on these lines, S. R. Bennett[2], using a thermo-electric pyrometer which had been previously standardized and its rate of error ascertained, and checking the results in some cases by a mercury-in-glass thermometer (the bulb of which was protected by metal tubing), determined the temperature of the various pots and baths of molten lead used in the Sheffield district. As was anticipated, temporary cessation of work, stirring up of metal, recoking of furnaces, and other causes, produced fluctuations of temperatures from minute to minute in the same pot, and in its different parts. The compensated pyrometer used gave for file-hardening pots a maximum of 850° C., and a minimum of 760° C., the average mean working temperature being about 800° C. The variations of temperature of lead used for tempering tangs of files and rasps was found to be high, and largely unrestricted from a practical standpoint. The maximum was 735° C., and the minimum 520° C., the average mean working temperature being 650° to 700° C., varying more than this within a few hours in the same pot. Spring tempering is carried out at some comparatively constant temperature between a maximum of nearly 600° C. and a minimum of 410° C., depending on the kind of steel and the purpose for which the steel is to be employed. Generally, the temperature required rises as the percentage of carbon in the steel is diminished. As these baths are larger than file-hardening pots, the temperature range is higher at the bottom than at the top unless well stirred up. Some lead pots are set in one side of a flue, and the temperature in the mass is then greater on the furnace side. From further observation of these pots during experiments, he was inclined to believe that the lead did not volatilize directly into the atmosphere, as heated water does, but that the particles of coke, fused oil, etc., which rise from the surface, act as carriers of the rapidly oxidized lead particles which cling to them.
Similar experiments were carried out in letterpress printing works. The average temperature was 370° C. in the stereo pots, and in the linotype pots at work 303° C. Scrap lead melting-pots when hottest registered 424° C., but registered as low as 310° C., according to the amount of scrap added, the state of the fire underneath, etc. The best practical working temperature depends largely on the composition of the metal used. That at some factories is the same for stereo drums as for lino pots—viz., 81·6 per cent. lead, 16·3 per cent. antimony, and[202] 2·0 per cent. tin, added to harden the lead. On the other hand, some printers use a higher percentage of antimony in the lino than in the stereo metal. Lead melts at 325° C., and antimony at 630° C., but by adding antimony to lead up to 14 per cent. the melting-point is reduced at an almost uniform rate to 247° C., after which further addition of antimony raises the melting-point. This explains why temperatures as low as 290° C. are practicable for linotype pots. The molten eutectic has a specific gravity of about 10·5, whereas the cubic crystals average 6·5 only; therefore in these pots the latter float on the top, and excess of antimony is to be expected in the skimmings or on the surface.
Administration of certain sections of the Factory and Workshop Act, 1901, would be simplified were there a ready means available for determining the extent of contamination of the air—especially of Section 1, requiring the factory to be ventilated so as to render harmless, as far as practicable, all gases, vapours, dust, or other impurities, generated in the course of the manufacturing process, that may be injurious to health; of Section 74, empowering an inspector to require a fan or other means if this will minimize inhalation of injurious fumes or dust; of many regulations having as their principal object removal of dust and fumes; and of Section 75, prohibiting meals in rooms where lead or other poisonous substance is used, so as to give rise to dust or fumes. Unfortunately, owing to the difficulty hitherto of accurate collection, only a very few determinations of the actual amount of lead dust and fume present in the atmosphere breathed have been made. This lends peculiar value to a series of investigations by G. Elmhirst Duckering, which have thrown much light on the amount of lead fume present in the air of a tinning workshop, and the amount of lead dust in the air during certain pottery processes, and the process of sand-papering after painting. Incidentally, also, they help to determine the minimal daily dose of lead which will set up chronic lead poisoning[3]. Aspirating the air at about the level of the worker’s mouth for varying periods of time, he determined the amount of lead in the fume, or in the dust, per 10 cubic metres of air, and from knowledge of the time during which inhalation took place he calculated the approximate quantity inhaled per worker daily. We have summarized some of his conclusions in the table on pp. 204, 205:
[203]
Duckering’s experiments as to the presence of fumes containing compounds of lead in the atmosphere breathed were carried out in a workshop for the tinning of iron hollow-ware with a mixture consisting of half lead and half tin. The process of manufacture and the main sources of lead contamination in the air (knowledge arrived at from these experiments) are explained on p. 59. As the result of laboratory experiments designed to show the effect of the violent escape of vapour produced below the surface of molten metal in causing contamination of the air, and the nature of the contaminating substances, he was able to conclude that the chemical action of the materials (acid and flux) used, and subsequent vaporization of the products of this action, was a much more important factor than the mechanical action of escaping vapour. Subsequently, experiments carried out on factory premises gave the results which are expressed in the table as to the relative danger, from lead, to (a) a tinner using an open bath; (b) a tinner working at a bath provided with a hood and exhaust by means of a furnace flue; and (c) the nature and extent of air contamination caused by the operation of wiping excess of metal (while still in a molten state) from the tinned article. In all three experiments aspiration of air was made slowly: it was maintained at the rate of 3 to 4 cubic feet an hour in the first experiment for between seven and eight hours; in the second for twenty-eight to twenty-nine hours; and in the third for twenty-four to twenty-five hours. The person engaged in tinning at the open bath was shown to be exposed to much more danger than one working at a hooded bath, while the wiper was exposed to even more danger than the tinner using an open bath, since not only was he inhaling fume from the hot article, but also fibre to which considerable quantities of metallic lead and tin adhered.
Analysis of samples of dust collected in different parts of the workroom bore out the conclusions derived from analysis of the fumes. Thus, samples collected from ledges at varying heights above the tinning bath containing the mixture of tin and lead contained percentages of soluble lead (lead chloride) in striking amount as compared with samples collected at points in the same room remote from any source of lead fume, while the insoluble lead present, as was to be expected from the fact that it consisted of lead attached to particles of tow floating in the air, was less variable.
[204]
TABLE XII., SHOWING QUANTITIES OF LEAD (Pb) IN THE ATMOSPHERE AT BREATHING LEVEL.
(G. E. Duckering’s Experiments.)
[206]
—Reference to the table shows that the conditions in the pottery workrooms, as stated in Column 7, are reflected in Columns 3 and 5. Further details from his experiments may be useful. Thus, in a dipping room where low-solubility glaze was in use, the amount of lead in the dust collected per 10 cubic metres of air was 0·70 milligramme. The average of four experiments where there were no dipping boards was 1·80 milligrammes, and where dipping boards were used, 3·75; i.e., 1·95 milligrammes of lead in the dust per 10 cubic metres of air is added by the use of dirty dipping boards. As the result of his experiments, Duckering believes that approximately 1·95 milligrammes of lead per 10 cubic metres of air was due to the fine spray given off in the shaking of the ware. In bright sunlight, he says, the spray can be seen dancing high above the dipping tub. In a dipping house where work was done slowly by two occupants only, the proportion of lead in the measured quantity of air was also low—0·58 milligramme per 10 cubic metres. Where, in the absence of special provision made for admission of fresh air to a fan, the air was drawn from a neighbouring room in which lead processes were carried on, the amount of lead rose to 5·76 milligrammes at the level breathed by the gatherer at a mangle. In ware-cleaning the average of all his observations where lead was used (eleven) was 3·44 milligrammes; and he concluded that “wet cleaning of ware causes less direct contamination of the atmosphere, even where no local exhaust is applied. A still more important result of wet cleaning, however, is that the overalls keep much freer of dust.” The highest results were obtained when the process of ware-cleaning was done outside the influence of the exhaust draught. In one instance, where the ware was cleaned at a distance of 6 feet from the exhaust opening, 13·34 milligrammes per 10 cubic metres of air were found. Subsequently at the same point, after the exhaust system of ventilation had been remodelled, 0·95 milligramme only was present. Even in a stillage room in which no work was done other than the placing on and removal of the boards from the racks, the lead content per 10 cubic metres of the air was 1·08 milligrammes. In glost-placing, the average of four experiments was 1·83 milligrammes—no doubt the result of glaze on the boards. As much as 9·11 milligrammes of lead was found per 10 cubic metres of air in the centre of a large majolica-painting room, with wooden floors and much traffic in it. Wooden floors generally appeared to influence the[207] results, as determinations of the lead present were higher in rooms with them than with tiled floors.
In coach-painting the proportion of lead found by Duckering in the air breathed during the actual time of sand-papering explains the severe incidence of poisoning in this class of work. The table shows the amount of lead in the air to be enormous, and in many cases much in excess of the amount found in the air when wiping off in the tinning of hollow-ware. The work of sand-papering is, however, very rarely continuous, the time occupied in it being, for the painter, about one to two hours daily; for the brush hand, two to three and a half hours; and for the painter’s labourer, four to five hours.
Knowing intimately the processes at which the estimations recorded in the table were made, the relative frequency of cases of plumbism reported among those employed at them, and the duration of employment prior to attack, we believe that, if the amount of lead present in the air breathed contains less than 5 milligrammes per 10 cubic metres of air, cases of encephalopathy and paralysis would never, and cases of colic very rarely, occur. And this figure is a quite practical one in any process amenable to locally-applied exhaust ventilation. Somewhere about 2 milligrammes, or 0·002 gramme, of lead we regard as the lowest daily dose which, inhaled as fume or dust in the air, may, in the course of years, set up chronic plumbism.
—In considering preventive measures against lead poisoning, precedence must be given to removal of fumes and dust by locally-applied exhaust ventilation, as, unfortunately, the wearing of a respirator is neither in itself a sufficient protection, nor, if it were, could the constant wearing of one be enforced. A respirator is of no use against lead fume. In the case of dust, the conditions which it must fulfil to be effective are, first, that the air breathed is freed from dust, and, secondly, that it should not incommode the wearer. Further, it should be simple in construction, easily applied, and allow of frequent renewal of the filtering medium. No existing respirator of moderate price conforms quite satisfactorily with these requirements. The more closely to the face it is made to fit, and the more effectually the air is filtered, the greater is the inconvenience experienced when it is worn. This inconvenience is due to the exertion (showing itself in increase of the respiratory movements and pulse-rate) caused in aspirating the air through[208] the filtering medium, and rebreathing some portion of the expired breath, containing a much greater proportion of carbonic acid gas and of moisture at a higher temperature than are present in fresh air. Respirators, therefore, except for work lasting a short time—half an hour to an hour—cannot be considered an effective or sufficient means of protecting the worker against dust. If a respirator must be worn, the simplest form is a pad of ordinary non-absorbent cotton-wool (absorbent wool quickly becomes sodden and impervious), about 3 inches by 4 inches, placed over the mouth and nostrils, and kept in position by elastic bands passed round the ears. The pad should be burnt after use.
With a smooth, impervious floor, however, and ventilation designed to remove the fumes and dust at, or as near as possible to, the point of origin, lead poisoning would become very rare in most of the industries to be described. The essential points of such a system are—(1) The draught or current of air set in motion either by heat or by a fan; (2) the ducts along which the current travels; (3) the hoods or air-guides designed to intercept and catch the fumes and dust at the point of generation; (4) inlets from the outside air into the room to replace continuously the air extracted, and, in many cases, (5) a suitable dust filter or collector.
—Processes giving rise to fumes or to dust liberated on stirring or skimming, which can be dealt with by the draught created in the furnace flue or over a bath of molten metal provided with adequate hood and duct up which the heated air travels, are—Smelting, refining, spelter manufacture, and the numerous operations necessitating the melting of lead, such as tinning with a mixture of tin and lead, sheet lead and lead piping, stereo pots in letterpress printing, pattern-making, tempering springs, file-hardening, etc. The dusting of red-hot metallic surfaces, as in vitreous enamelling, might possibly also be dealt with in the same way. The disadvantage of the exhaust by heat is the uncertainty and inequality of the draught, and the size of the duct necessary to cope with the volume of rarefied air from above the molten vessel.
The closer the hood is brought down over the point where the fumes escape, the less risk is there of cross-currents deflecting them into the workroom. Hence all baths of molten metal should have the sides and back closed in, leaving as small a space open in front as is practicable in view of necessary skimming or other operations.
[209]
In the case of tinning baths, Duckering[4] describes completely successful results when from the top of the hood a shaft at least 24 inches in diameter was carried vertically upwards into the open air to a height of 18 feet, and the top of the shaft fitted with a wind screen in the form of a very large cone, having its lower edge below the upper edge of the shaft, and its nearest point at least 8 inches from the top of the shaft. Smoke produced in large quantity at any point 6 inches outside the front of the hood was entirely drawn into it. As, however, the inrush of air caused an eddy of the fumes at the upper edge of the opening, the edges of the hood were turned inwards, so that the operation of wiping was done in a sort of short tunnel. In general, it may be said that the diameter of pipes leading from hoods to the outer air (on the efficacy of the draught in which success depends) is much too small. Frequently mere increase in size will convert an indifferent draught into a good one. The height of the hood also—i.e., the distance between its lower border and the point where it joints the duct—is of importance. The shorter this distance is, the less serviceable does it become for the removal of fume. Indeed, it may even retain the fume which, were the hood not present, would rise to the roof. Sometimes safety is increased by making the hood double, leaving a space between the two sheets, and so concentrating the draught at the centre and at the margin. With a fan, ducts of less diameter can be used than when dependence is placed on heat alone. A duct carried into a chimney-stack has the advantage of dispersing the fume at a safe distance from the workroom.
The variableness of the draught produced by heat makes it unsuitable for removal of dust, except such as arises from skimming. The receptacle for the skimmings should always be kept inside the canopy of the hood. We have, however, seen the dust given off in the heading of yarn dyed with chromate of lead successfully carried away under hoods connected up by branch ducts with the main chimney-stack.

Fig. 1.—Davidson’s Sirocco Propeller Fan.
—The draught for removal of dust, and frequently also of fumes, is produced by a fan, of which there are two types: (1) low-pressure volume fans and (2) high-pressure centrifugal fans. In the first the draught is created by the rotation of a wheel with inclined vanes, causing the air to be driven transversely through the wheel parallel to the axis of rotation (Fig. 1). During a revolution a portion of the air is[210] cut off from one side of the wheel, and transferred through the wheel to the other. Such fans are light, run easily, and are cheap. They are of many forms, both with regard to the number of blades—from two to eight—and general manner in which they are arranged. Some closely resemble the screw-propeller of a ship, while others have blades turned over and fastened on an outer rim. Their main defect is inability to overcome any but slight resistance in the course of suction behind, as from constriction in, or friction along the sides of, the ducts and right-angled bends, or of outflow in front, as from wind-pressure. Under favourable conditions, however, and when carefully fitted, a volume fan will exhaust dust and fumes through a system of ducts several feet in length, as, for example, from mono and linotype machines and electro melting-pots in letterpress printing works. But, in order to avoid resistance from friction, the ducts have to be somewhat larger in diameter than when a centrifugal fan is used. With nine[A] linotype machines connected up to a 14-inch propeller fan, the branch ducts should be about 4 inches in diameter, and the main duct 12 inches, increasing from 12 to 15 inches within 2 feet of the fan-box. The shorter and straighter the course of the duct to the propeller fan, the more efficiently it works. Wind-guards are necessary to overcome resistance from[211] this source in front, but their position requires to be carefully considered, so as to prevent the screen itself crippling the outflow.
[A] If gratings are also inserted in the same duct for general ventilation the number of machines must be decreased pro ratâ.
All fans require frequent cleaning, and in this respect propeller fans have the advantage over centrifugal, in that they are usually more accessible.
Fig. 2.—Davidson’s Dust Centrifugal Fan.
—Generally, in the removal of dust, a strong suction has to be set up in a system of narrow ducts by means of a centrifugal fan—i.e., a fan-wheel formed by a number of vanes attached to an axle mounted in a spiral-shaped casing—so that when the wheel rotates air is carried along by the vanes, and flies off tangentially into the space between the blades and the casing, and thence to the outlet (Fig. 2). The air inlet or junction of the fan with the exhaust duct is at the centre of the fan, an arrangement by which the kinetic energy created by the rapid motion of the air leads to increase of draught instead of being wasted in production of eddies in the surrounding spaces. They are made in many different patterns, according to the[212] nature of the work to be done. Their advantage over the propeller type in the removal of dust lies in the fact that they overcome greater internal resistance, and a uniform high velocity in a complicated system of pipes can thus more easily be maintained.
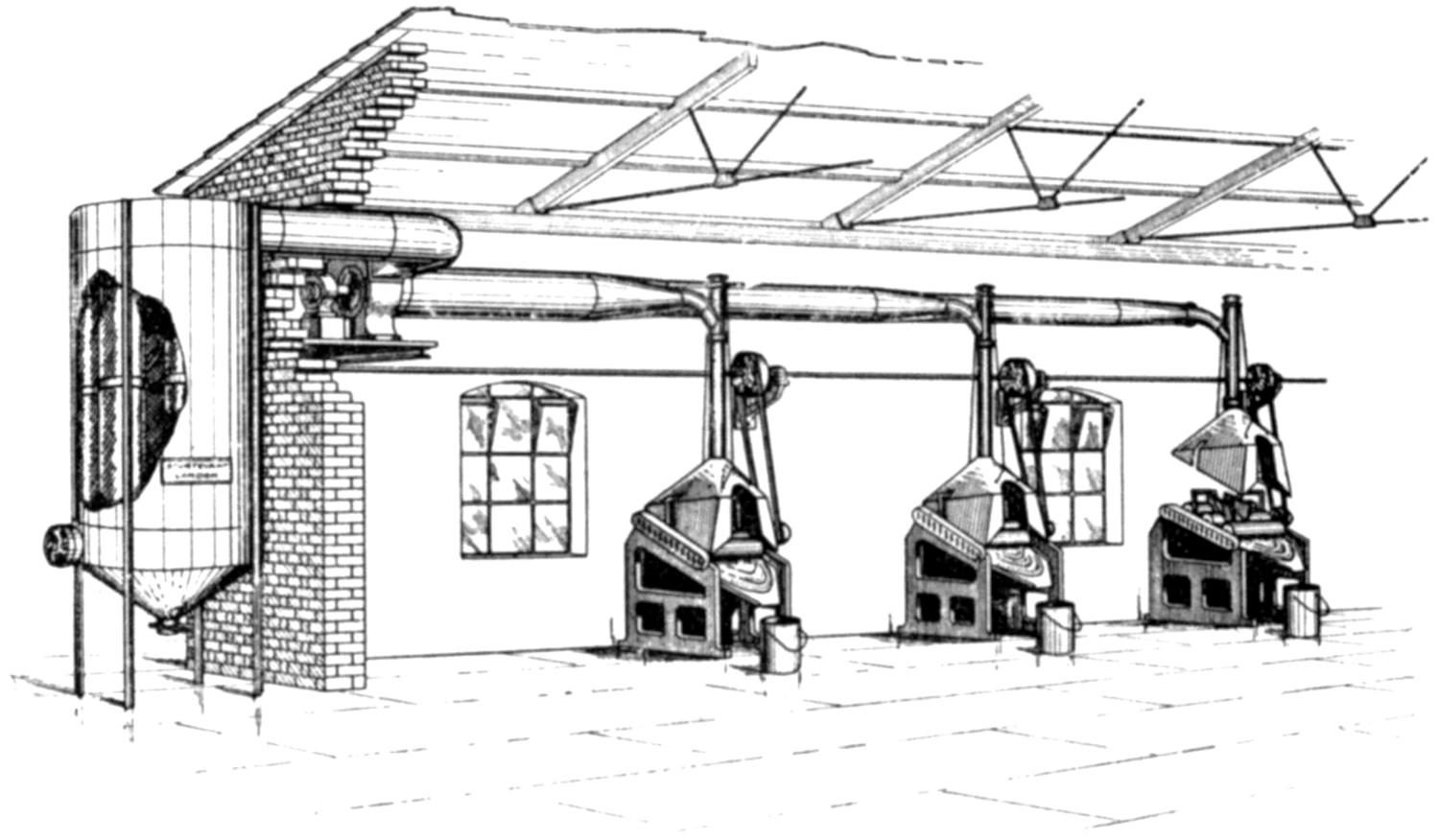
Fig. 3 shows adjustable hoods and ducts fitting closely over rollers for mixing coloured inks, and serving not only to prevent inhalation of lead dust by the workers, but also the colour from one machine affecting that on another. In the particular room where the installation is fitted there are thirteen separate sets of rollers; the diameter of the branch duct of each machine is about 5 inches, and that of the main duct close to the fan about 20 inches. The special points we have considered as to entrance of all branch ducts into the main duct tangentially, gradual tapering of the main trunk, and collection of the dust in filter-bags, are noticeable. Further, when one set of rollers is not in use the raising of the hood automatically cuts off the draught through it. (Drawing supplied by the Sturtevant Engineering Company, Limited, London.)
—The main duct should be of metal (steel, sheet-iron, or zinc); it should be circular in shape, have as straight and short a course as possible, and be tapered in such manner that the area of cross-section at any point shall equal the combined areas of all the branch pipes which have entered it at that point (Fig. 3). Proper dimensions must be studied in relation to the size of the fan and the work to be done. Wooden ducts, unless chosen for specific reasons, such as the presence of acid in the fumes to be removed, are very unsatisfactory, as it is difficult to maintain them in an air-tight condition or to make branch pipes enter with rounded junctions. Where several branch ducts enter a main duct, situation of the fan midway between them has advantage, not only in saving metal in piping, but also in causing the distance of the fan from the farthest branch[213] duct to be only half what it would be were the fan placed at the end of the system (see Fig. 7, p. 217). Further, the sectional area of the two collecting ducts will be less than that of one main duct, and greater uniformity of flow thereby secured. Where the two ducts join up into the single duct of the fan, the bends must be easy; otherwise the draughts would collide and neutralize one another. Branch ducts, if they cannot be made tangential to a rounded curve, should enter the main duct at an angle of 30 degrees, as by so doing equalization of the draught at different openings is made fairly uniform. The very common defect of a right-angle joint diminishes the draught by nearly one-half. Branch ducts should never be made to enter a main duct on the outer side of a bend, because at this point the pressure of the current of air inside the duct is increased. They should join up on the inside of a bend, where the pressure is reduced.
—As the object of hoods is to concentrate the draught on the fumes or dust to be removed from the worker, position in regard to origin of the fumes or dust requires first consideration. The more restricted the opening consistent with unimpeded work, the more effective is the draught, and the less disturbed will it be by cross-currents in the workroom. Pendock lays it down as a useful principle that the area of the front opening into the hood should not be more than four times that of the exhaust throat—i.e., the point of junction of the hood and duct (Fig. 4). Not less important is it that the draught should operate below the breathing level. Preference as to the direction to be given to the exhaust current should be in the order named: (1) Downwards; (2) downwards and backwards combined; (3) backwards and upwards combined; and (4) upwards only. Use should be made, for the removal of the fumes or dust, of any initial current of hot air set up from a bath of molten metal or from a heated metallic surface, as in vitreous enamelling. Hence under such circumstances only (3) and (4) need be considered. Generally hoods applied err in having too wide an opening, or they are placed too far away from the source of danger. They require sometimes to be adjustable to suit different-sized articles. Care is necessary to see that, when a hood has been adjusted for large articles, it is readjusted for smaller-sized articles. The principle of ventilation downwards and backwards is recognized as right for grinding and polishing on a wheel, since the tangential current set up by the wheel in its rotation[214] is utilized. Pug-mills in paint-works are perhaps best ventilated by applying the exhaust to a dome-shaped hood covering the posterior half of the mill. Edge-runners must be encased, with an exhaust pipe attached to the casing and sliding doors or shutters for introduction or removal of material (Fig. 5). A small negative pressure inside the casing is all that is necessary, so as to insure passage of air inwards and not outwards. Branch ducts must protect the casks out of which material is scooped, and the receptacle into which it is discharged. In scooping out dry colour from a barrel, it is unwise to attempt to remove the dust created at every displacement of air on removal of a scoopful by means of a hood suspended over the barrel. Instead, the last joint of the duct should be a telescopic one, so that it can be lowered into the barrel, and be kept at a distance of about 6 inches above the material. The air is thus drawn downwards into the barrel (Fig. 6).
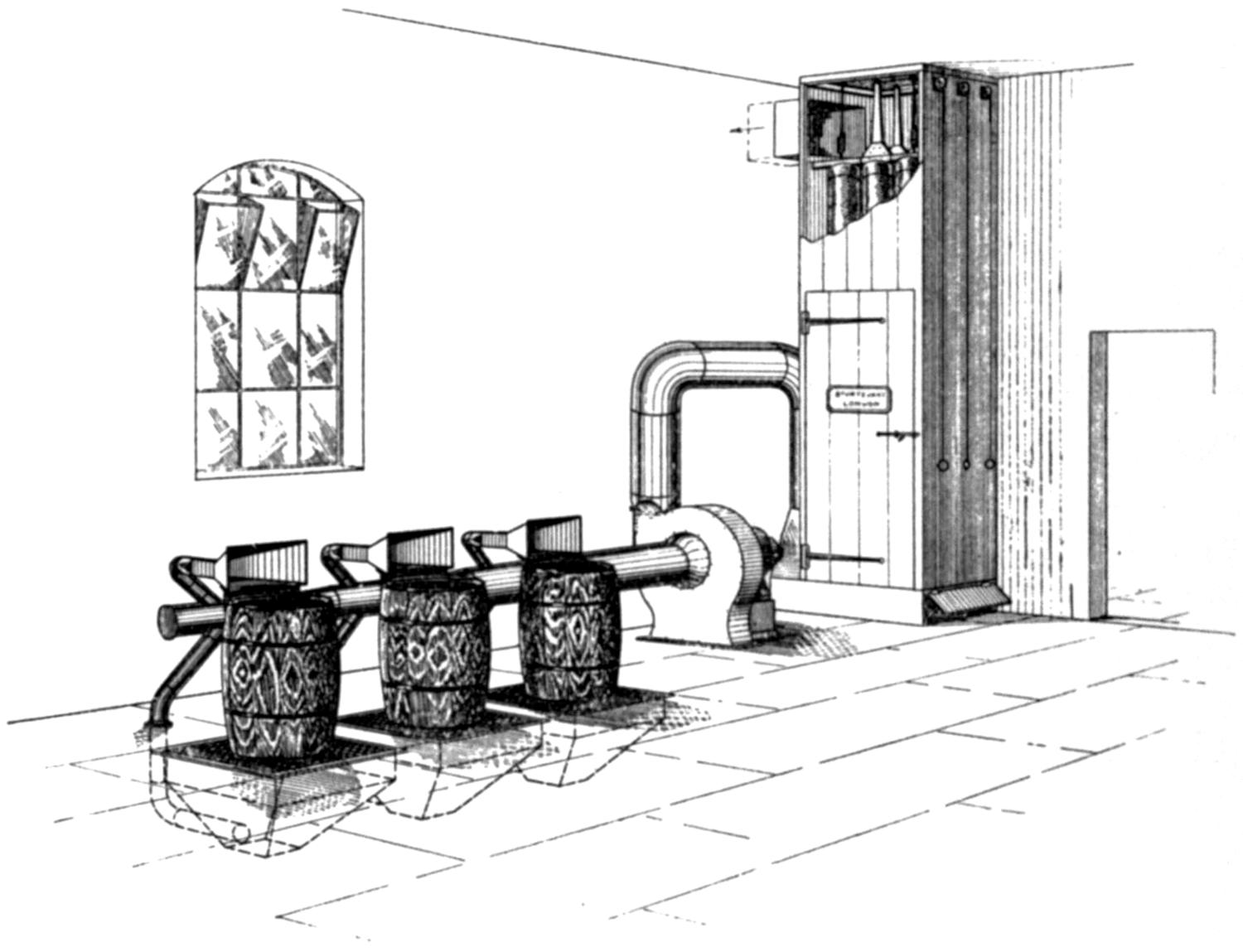
Fig. 4 shows a well-designed arrangement of hoods, duct, and fan, in the packing of white lead, and the filter-bags for collecting the dust so removed. An additional safeguard is introduced, as the casks stand upon grids through which a down-draught is maintained by connecting the space underneath with the exhaust system. (Drawing supplied by the Sturtevant Engineering Company, Limited, London.)
Processes such as colour-dusting, aerographing, ware-cleaning,[215] enamel-brushing, and the like, are best carried out at benches under hoods with glass tops. Air will enter from in front, and carry the dust or spray away into the exhaust duct placed at the back of the bench.
Fig. 5 shows a pan mill with edge runners fitted with casing (partially open). The casing is connected to a powerful fan, and branch ducts with telescopic terminal sections control the dust in scooping out from the barrel, in feeding into the mill, and at the point where the ground material is discharged.
—Frequently no heed is paid to the collection of the dust. Sometimes a dust chamber is arranged to intercept it on the far side of the fan, or attempt is made to[216] blow the dust into a tank of water. The fine dust of which we are speaking cannot be satisfactorily collected by either of these methods, nor even by a cyclone separator, so useful for the collection of many kinds of dust. In lead works generally, the dust removed by the fan is best collected in filter-bags made of some porous fabric. Various efficient filters constructed on these lines by Messrs. Henry Simon, Ltd.; Messrs. Beth and Co., Ltd.; and the Sturtevant Engineering Company, Ltd., are on the market.
Fig. 6 shows an arrangement of piping with balanced telescopic joints fitted to a Sirocco dust fan for removal of dust, in an electric accumulator works, when scooping out litharge from a cask into the receptacle prior to emptying the weighed quantity into the mixing machine, also under a hood connected with the exhaust system. (Illustration supplied by Davidson and Company, Limited, Belfast.)
In collecting the dust, care must be taken to provide an adequate outlet for the spent air, so as to prevent creation of a source of friction in front which might destroy the effectiveness of the installation.
[217]
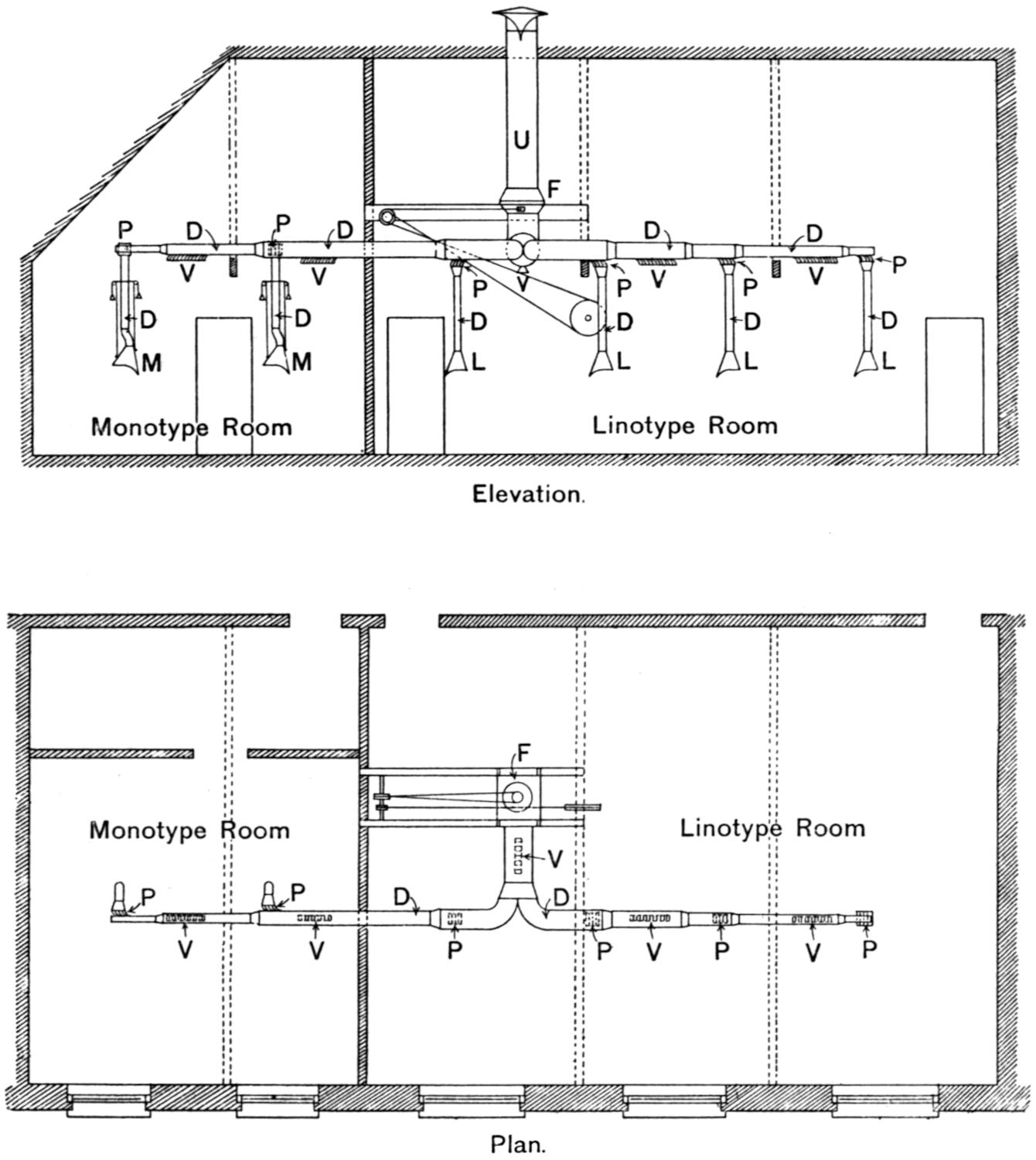
Fig. 7.—Exhaust Ventilation on the Patent “Pentarcomb” Principle applied to Linotype and Monotype Machines in Printing Works, as installed by the Zephyr Ventilating Company, Bristol.
P, Patent “pentarcomb” for equalizing exhaust; V, patent “pentarcomb” for general ventilation; D, main and branch ducts; F, fan; U, upcast from fan; M, hoods over metal-pots of monotype machines, constructed to raise and lower, and swing out and in with metal-pot; L, hoods over metal-pots of linotype machines, constructed to raise and lower.
In the illustration “pentarcomb” grids connect the branch ducts over the metal-pots of mono and linotype machines with the main duct. The “pentarcomb” grids are arranged also elsewhere in the main duct itself to assist in the general ventilation of the workroom. The hoods over the metal-pots are constructed to be raised and lowered, and to swing out and in radially with the melting-pot arm. (Drawings supplied by the Zephyr Ventilating Company, Bristol.)
In order to secure equality of flow from a number of branched ducts, the Zephyr Ventilating Company apply a special grating of curved and slanting inlets—the “pentarcomb”—to each branch[218] duct. The air passing through the comb is split up into numerous small columns, and the inclination of the curve which each is made to take is such as to reduce friction to a minimum. By means of this device we have found, in a trunk with twenty branches, the draught at the one farthest from the fan as serviceable as that next to it. The method is illustrated applied locally to remove the fumes from linotype machines, and generally in the main duct for removal of foul air near the ceiling.
Where electricity is available as a motive power for driving the fan, some modification in the views expressed as to the curvature of the pipes and system of installation can be allowed. In a red lead plant, for instance, it may be desirable to have the pipes leading to the sifter or packing machine with sharp angles, so as to prevent tendency of such heavy dust to collect in them. The electric current allows a fan to be installed at any point desired; and if applied with knowledge that the increased friction due to an acute angle has to be overcome, the result may be quite satisfactory.
The various forms of vacuum cleaning apparatus with mouthpieces designed to aspirate the dust from different surfaces are sure to be increasingly used. In our opinion, wherever electric power is available, they will obviate barbarous methods involving use of hand-brushes to collect dust from machines, such as those for litho-dusting or for sweeping lead dust from benches and floors, or use of bellows to blow out the dust from compositors’ cases.
Finally, the carrying out of lead processes by automatic methods and with the interior of the casing under a negative pressure, so that the material is transported from one process to another by means of worms or conveyors, is everywhere to be aimed at. Or, again, it has been found possible on a commercial scale, by means of compressed air in a closed system of receivers and pipes, to force material in very fine state of division from one place to another, as, for instance, of litharge from the cask into the mixing machine for preparation of the paste for manufacture of accumulator plates, without risk of contact.
Indication of the efficiency of the draught may be gained by holding smoke-paper at the orifice of the hood. The definition of efficient exhaust in some regulations for the removal of fumes, as in the Tinning Regulations, is that it shall not be deemed to be efficient unless it removes smoke generated at the point where[219] the fume originates. Accurate gauging, however, of the draught can only be done with an anemometer, so as to determine the number of linear and cubic feet passing through the throat per minute. Only rarely does one find an occupier alive to the value of the use of such an instrument. The importance of this point has been recognized in the Regulations for Heading of Yarn, by the requirement that the speed of each exhaust opening shall be determined once in every three months at least, and recorded in the general register. We prefer to use Davis’s[A] self-timing anemometer, which gives readings in feet per second without the need of a watch. Other useful anemometers—Casella’s or Negretti and Zambra’s—require to be timed.
[A] It is not available for velocities exceeding 1,200 linear feet per minute.
The details of all routine observations on localized exhaust ventilation might well be entered on a card hung up in the workroom. Such a card drawn up by our colleagues, Miss Lovibond and Mr. C. R. Pendock, has the following headings:
[220]
Frequent cleaning and inspection of exhaust installations are very important, as accumulation of dust greatly impedes the flow of air at all points of the system. The person employed in cleaning the fan should wear a respirator. Hoods and ducts should always be cleaned with the exhaust in full action.
[1] Annual Report of the Chief Inspector of Factories for 1910, p. 172.
[2] Ibid., pp. 172, 173.
[3] G. Elmhirst Duckering: A Report on an Experimental Investigation into the Conditions of Work in Tinning Workshops, and Appendices. Included in Special Report on Dangerous or Injurious Processes in the Coating of Metal with Lead or a Mixture of Lead and Tin. Cd. 3793. Wyman and Sons, Ltd. Price 1s.
G. Elmhirst Duckering: The Cause of Lead Poisoning in the Tinning of Metals. Journal of Hygiene, vol. viii., pp. 474-503, 1908.
G. Elmhirst Duckering: Report on an Investigation of the Air of Workplaces in Potteries. Included as Appendix XLIX. in Report of the Departmental Committee appointed to inquire into the Dangers attendant on the Use of Lead, and the Danger or Injury to Health arising from Dust and Other Causes in the Manufacture of Earthenware and China, vol. ii., pp. 93-113, 1910. Cd. 5278. Price 1s. 9d.
[4] G. Elmhirst Duckering: Annual Report of the Chief Inspector of Factories for 1910, p. 47.
[5] C. R. Pendock (one of H.M. Inspectors of Factories): Report on Systems of Ventilation in Use in Potteries. Included as Appendix XLVIII. in vol. ii. of Potteries Committee’s Report referred to under[3].
C. R. Pendock: Second Report of the Departmental Committee appointed to inquire into the Ventilation of Factories and Workshops, part i., and especially part ii., 1907. Cd. 3552 and 3553. Price together, 4s. 8d.
Other works referred to include—Construction des Usines au Point de Vue de l’Hygiène, by Ingénieur-Architecte Maniguet. Ch. Béranger, Paris, 1906; Hygiène Industrielle, by MM. Leclerc de Pulligny, Boulin, and others. J. B. Baillière et Fils, Paris, 1908; and many excellently illustrated trade catalogues issued by ventilating engineering firms, such as the Sturtevant Engineering Company, Ltd., London; Henry Simon, Ltd., Manchester; Davidson and Company, Ltd., Belfast; John Gibbs and Son, Liverpool.
[221]
—In various codes of regulations a surgeon is required to make periodical medical examination of the workers. The term “surgeon” is defined as the “Certifying Factory Surgeon of the district, or a duly qualified medical practitioner, appointed by written certificate of the Chief Inspector of Factories, which appointment shall be subject to such conditions as may be specified in that certificate.” The wording of the regulation varies somewhat in different codes, but the intention in all is the same, and the following example from the Tinning Regulations will indicate the purpose and scope:
“Every person employed in tinning shall be examined by the surgeon once in every three months (or at such shorter or longer intervals as may be prescribed in writing by the Chief Inspector of Factories), on a day of which due notice shall be given to all concerned. The surgeon shall have the power of suspension as regards all persons employed in tinning, and no such person after suspension shall be employed in tinning without written sanction from the surgeon entered in the health register.
“Every person employed in tinning shall present himself at the appointed time for examination by the surgeon. No person employed in tinning shall, after suspension, work at tinning without written sanction from the surgeon entered in the health register.”
Under the Special Rules for white-lead works, examination is required at weekly intervals; under the Special Rules for Earthenware and China, Manufacture of Litho-Transfers and Red Lead, and under the Regulations for Electric Accumulators, and Paints and Colours, monthly; under the Regulations for Tinning, Yarn dyed with Chromate of Lead, and Enamelling, at quarterly intervals, subject to the limitation or extension specified in the regulation quoted.
The limitation as to quarterly examination is useful to meet conditions, on the one hand, where special incidence calls for increased safeguards; and, on the other, relaxation, by reason of adoption of special processes or measures lessening risk. Thus,[222] in a yarn-dyeing factory, in consequence of occurrence of six cases within five months, a weekly instead of a quarterly examination was prescribed. After eight months, as no further cases were reported, a monthly examination was substituted for the weekly, and eventually, with continued absence of illness, the normal quarterly examination was resumed.
An appointed time for the surgeon’s attendance at the factory has been found necessary, because, in conformity with the literal wording of the regulation, the occupier should not continue to employ a worker who, for one reason or another, has not been examined by the surgeon during the prescribed interval. With knowledge of the date and hour posted in a conspicuous place in the factory, excuse for absence becomes difficult. Alteration by the surgeon of his appointed time should, whenever possible, be given beforehand. Surgeons in the past frequently made examination of the persons employed with the view of taking them unawares, and so of precluding special preparation beforehand—a practice which had its advantages; but they are outweighed by the hardship inflicted on workers who were unavoidably absent, as, for example, night-workers. A health register is supplied to all occupiers where periodical medical examination is enjoined, the headings of which and manner of entry are indicated later on in this chapter.
The objects which the surgeon should have in mind in making his examination are:
1. To prevent lead poisoning and minimize lead absorption.
2. To obtain information for the occupier and Inspector of Factories of the relative danger of one process and another with a view to adoption of remedial measures.
In safeguarding the health of the workers, he should make effort to gain their confidence, in order to be able to attach proper value to statement as to subjective symptoms. Suspicion in their minds that the examination is made solely in the interests of the employer militates against success, and increases inclination to conceal symptoms and to give untruthful answers as to the state of health since the last examination. In our opinion, the surgeon will best carry out the first object by attention to the second. The study of thousands of reports on cases of lead poisoning convinces us that 90 per cent. at least are due to inhalation of dust and fumes. The surgeon, therefore, should utilize the earliest sign of lead absorption to warn the occupier[223] and inspector of conditions favourable to the development of plumbism, and due probably either to some unguarded spot in the manufacturing process whereby dust or fumes are not being removed completely, or to ignorance or carelessness (often excusable in the absence of proper instruction) on the part of the worker. He should direct, therefore, especial attention to new workers, not only because of their need for guidance as to precautions to be observed and greater liability to attack during the first year of employment, but also because development of signs in them constitutes the surest guide to defects in the process of manufacture. Occasionally symptoms in a worker may be so menacing as to demand immediate suspension, but generally before the power is exercised attempt to rectify the condition which gives rise to them should be made. The surgeon can do much by influencing the foremen and forewomen, who will necessarily come before him for examination, in insisting on the supervision by them of care and cleanliness by the workpeople under their charge. Should suspension, despite attention in the manner suggested, be necessary, he will recognize that transference to a non-lead process, if feasible, is preferable to entire cessation from work in very many cases. The surgeon, therefore, should know what departments are possible alternatives to lead work.
The fact that an examination is made on factory premises, is directed to detection and prevention, treatment taking a subordinate place, and is often made on persons who, unlike hospital patients, seek to conceal their symptoms, causes it to be an examination sui generis. Hence the surgeon must trust his sight more than his hearing. A surgeon with experience of such work has said: “The worker in lead must be surveyed as an individual, and idiosyncrasies must be carefully studied and allowed for; the ‘personal equation’ is of vital importance”[1].
For the examination a well-lighted room affording privacy is essential. While it is desirable for the surgeon periodically to see the processes and conditions under which work is carried on, systematic examinations of workers should not be made elsewhere than in a private room. The custom of marshalling workers in a queue, although perhaps unavoidable in many cases, is liable to detract from the seriousness of the proceedings, a sense of which it should be one of the aims of the examination to arouse. In discussing the method of interrogation and usual examination, Dr. King Alcock[2], Certifying Factory Surgeon of Burslem,[224] says: “Note the general manner assumed in answering questions and any indications of carelessness in dress and toilet. Inquire into the state of digestion, existence of colicky pains, regularity of bowels, menses, history of pregnancies and miscarriages, whether before, in the intervals of, or during lead employment; existence of headache, diplopia, or amaurosis. Note the type, facies, state of teeth and nails, complexion, speech, tongue, strength of grasp (if possible, with dynamometer), any tremor in outstretched hand, resistance to forcible flexion of wrist.... If strabismus is present, note whether of old standing or recent; and if ocular troubles seem imminent, examine for optic neuritis, either at once or at home (this is very important, as cases of acute and serious optic neuritis still baffle examination by their intermensual development).” He recommends the surgeon, apart from entry in the health register, which must necessarily be very brief, to keep a private notebook, and to enter in it as a matter of routine such details as name, process, age, duration of employment, condition (married or single), pregnancies, state of bowels and menses, dental toilet, and any special point worthy of note in individual workers. A card index, if in use, might conveniently serve for such entries.
In the actual routine examination it may be useful to describe the procedure where a large number of workers pass before the surgeon in a white-lead works every week. The points noted are:
1. The general appearance of the man as he walks forward, especially the face with regard to anæmia, which in the majority of cases of early lead absorption is not a true anæmia, but is due to vaso-motor spasm of the arterioles of the face and eyes. Frequently, on speaking to a lead-worker, the face, apparently anæmic, flushes directly.
2. The brightness of the eyes, state of the pupils, and condition of the conjunctiva and of the ocular muscles.
3. The mouth should next be examined, and search made for any evidence of blue line around the gum.
4. The gait should be watched both on advancing to, and retiring from, the surgeon. If necessary, the man should be made to walk a few steps. Although the peroneal type of palsy is extremely rare, the possibility of its occurrence should never be absent from the mind of the surgeon.
5. The man should then be directed to stretch his hands out in front of him, with wrists extended and fingers widely spread.[225] Presence or absence of tremor should be looked for, and the condition of the finger-nails, as to the practice of biting, etc. The extensor power should then be tested, firstly of the fingers. While the hands of the workman remain outstretched, the surgeon places the forefinger of his hand in the outstretched palm of the workman, and the ball of the thumb upon the extreme tip of each finger, and by gently pulling it down, noting the spring present in the muscles. This test is probably the most delicate there is for detection of early extensor paralysis. The condition of the lumbricals and interossei are noted on movement of the fingers. The extensors of the wrist are then further examined, the workman being directed to flex his arm at the elbow and strongly pronate the wrist, so that the palm of the hand is directed forwards. He is then told to close the fist when the surgeon endeavours to flex the wrist, the workman at the same time resisting by forcible extension of his wrist. Ordinarily the extensor communis digitorum and minimi digiti are sufficiently powerful to resist a very powerful pull upon the wrist; and if the wrist is found to yield, it is a sign that the muscles are affected. Sometimes the strength of the wrists and fingers is judged by the surgeon placing his palms on the dorsum of the patient’s outstretched hands, and seeing whether the patient can be prevented from lifting them without flexing the wrists or finger-joints.
The test detects (1) paralysis which has been recovered from to a large extent; (2) commencing partial paralysis; and (3) weakness of muscular power, especially in those who have worked in lead for a number of years. This weakness appears to be an effect of lead upon the muscular tissue or dependent on debility, the result of lead absorption, and independent of nerve implication. We have known the condition to remain unaltered for years, and also to undergo alteration, being at times absent for months together. Occasionally reports of definite paralysis refer to pre-existing weakness.
6. The pulse is next noted. The pulse-rate need not ordinarily be counted, but if it is either very slow or fast careful examination at the conclusion of the general inspection should be made.
It is well to make all these points before asking any questions. After they are completed inquiry as to regularity of the bowels, existence of pain or discomfort, would follow. The speech should be noted, as slurring or hesitating speech is occasionally associated with early lead poisoning.
[226]
All these points can be gone through quite rapidly, and at the conclusion of the general examination, if judgment is in suspension, careful examination in the routine medical manner should be made.
In some factories all new workers are examined by the surgeon before they commence work in dangerous processes. At any rate, a list of such persons should be given to the surgeon at his visit, as naturally the question of personal fitness for employment should be decided at his first examination. Conditions which should lead to rejection are tubercular disease of every kind, idiopathic epilepsy, all forms of mental disease or weakness (hysteria, feeble-mindedness, and neurasthenia), obvious alcoholism, women who are pregnant or who give a history of repeated miscarriages prior to work in lead, persons with marked errors of refraction unless corrected by glasses, kidney disease of all kinds, evidence of previous chronic saturnism, and bad oral sepsis. Special attention will have to be paid to casual labourers, and it should be the aim of the surgeon to discourage this class of labour in lead industries. Work under special rules or regulations requires to be carried out under strict discipline, and this it is extremely difficult to maintain on other than regular workers, who recognize the need for cleanliness and observance of regulations.
Other aids to diagnosis cannot be carried out as a matter of routine, but will necessarily be used in particular cases, such as ophthalmoscopic examination of the fundus, electrical reactions of muscles, analysis of the urine, and examination of the blood-pressure.
A few words may be added on the significance of the two commonest signs—the blue line and anæmia. It cannot be too strongly insisted on that presence of the Burtonian line on the gums is, as a rule, indicative of lead absorption, and not of lead poisoning. As a danger signal its value is immense, and hardly less so its value in clinching diagnosis in doubtful cases. Whenever the line is seen risk is imminent, and poisoning (not necessarily of the individual in whom it is pronounced) among the workers is inevitable in the absence of adoption of precautions. Unfortunately, careful dental toilet, which the surgeon will necessarily lay stress on, may prevent development, or the practice, when adopted, cause disappearance of the line after the lapse of a few months. Under these circumstances, the merest trace will have all the significance of the fully-developed line in[227] a worker neglectful of care of the teeth. Among new workers a commencing blue line should be strong evidence of the need for dust removal at some point in the process of manufacture. The line, in our experience, is dense in occupations giving rise to fumes or to dust of compounds of lead, but comparatively rare in those handling metallic lead or its alloys, as compositors, tea-lead rollers, solderers, and the like.
Some degree of pallor is so commonly met with in adolescence that it is the progressive development of the anæmia which the surgeon must especially watch for. As a danger signal, therefore, it has the same significance nearly as the blue line; but when lead absorption has affected the elements in the blood, progressive anæmia in new workers, attributable to the employment, and showing no tendency to improve after watching for a few months, is an indication for suspension or transference to other work. In older workers, with a duration of employment of five years or more, there may be a quasi-pathognomonic pallor which does not vary from year to year. In them it must be supposed that an equilibrium has been established, and development of other symptoms, such as tremor, wrist weakness, or albuminuria, becomes significant. Attention has already been directed to the distinct saturnine facies associated with anæmia, and characterized by loss of fat, particularly noticeable in the orbit and buccinator region of the face. “So far as the question of any worker’s suspension is concerned,” says Dr. King Alcock, “I prefer to make my instinctive primâ facie distrust of a saturnine pallor the basis for action. The pallor of plumbism cannot be summed up in hæmoglobin and corpuscular content; it is the expression of a complex toxæmia resulting from defective assimilation and excretion”[3].
The knowledge the surgeon should gain of the idiosyncrasies of the workers by his periodical examination will enable him to appraise at their proper value the nature and degree of the symptoms in notified cases.
Sometimes a rule is made that no lead-worker who has suffered from an attack of plumbism should be allowed to resume work. This we consider too harsh a measure. It may be true for painters, but when remedial measures, such as locally applied exhaust ventilation, can be applied, with consequent removal of the danger in the process at which the poisoning has arisen, prohibition of employment seems an unnecessarily drastic measure.
[228]
The health register in general use where periodic medical examination is required in pursuance of special rules and regulations is divided into two parts, in each of which entries by the surgeon are required at each visit.
In Part I. of the register the surgeon should, at the times of examination, enter the date at the head of one of the columns numbered 6 to 9; and in the space below, opposite the name of each person examined on that date, a brief note (see next page) of the condition found.
In Part II. he should again enter, in Column 3, the date of examination, with a statement of the total numbers examined on that occasion (Column 4); and in Column 5 any certificate of suspension from work, or certificate permitting resumption of work, and particulars of any other direction given by him, appending his signature in Column 6.
It is the duty of the occupier to enter in Part I. the following particulars with regard to each person examined: (1) Name in full (Column 2); (2) the process in which he or she is employed (Column 3); (3) age when first employed (Column 4); and (4) date of first employment in that process (Column 5); and these particulars, in respect of each person so employed, must be entered immediately on commencement of work in the process named.
[229]
Various methods of noting the state of health of the workers have been adopted. Use of the words “Good,” “Very fair,” and “Fair,” is common as indicating the state of general health, with special note in addition, often in the form of a symbol, of the presence and character of definite ill-effects. The object of the register, however, is to keep a record intelligible not only to the Certifying Surgeon, Factory Inspector, and occupier, but also to the workers. Entries, therefore, on a uniform system are desirable, taking account of the two aspects of the health of every lead-worker, which must be considered (a) that indicative of specific effects from the occupation, and (b) that of general health uninfluenced by the employment. With this in mind, the following system of entry in the health register has been adopted:
The entries should be made upon a uniform system, as below, indicating degrees of deviation from normal health, and distinguishing (by use of numerals) those attributable (or possibly attributable, in whole or part) to work in lead, from those not so attributable, for which latter letters should be used. The conclusion is perhaps best expressed as a fraction 1 A, 2 C, and so on.
The numerals should be taken to mean:
1. Passed without comment (no observed effect of lead).
2. Blue line (or indication thereof).
3. Marked (quasi-pathognomonic) anæmia, or other signs of impairment of health. (Albuminuria, or slight deficiency in tone of the extensor muscles of the forearm, would, and miscarriage, or other suspicious history of illness between examinations, might, come under this head.)
4. Suspension or transfer, by reason of impairment of health from effects of work in lead. (In such cases the surgeon would be prepared to entertain an application for a certificate under the Workmen’s Compensation Act.)
Except in the case of a worker whose exposure to lead is only recent, renal disease should always be indicated by a numeral.
Letters should bear the following meaning:
A. No comment (i.e., fair general health).
B., C. Increasing degrees of impairment of general health. (Pregnancy, if without suspension, should be entered as C.)
D. Suspension or transfer, for reasons other than impairment of health from effects of work in lead.
X. Carelessness, or neglect of precautions, or unsuitability for work in lead. (Suspensions for such reasons should be marked DX.)
Such entries of numerals and letters will in general suffice for the intended purpose; but the surgeon may, of course, find it desirable to make other notes for his own information, and it is within his discretion to supply further details to occupiers or workers concerned.
[1], [2] S. King Alcock, B. M. Bond, A. Scott, and others, in discussion on the Value of Systematic Examination of Workers in Dangerous Trades. Brit. Med. Journ., vol. ii., pp. 741-749, 1902.
[3] King Alcock: The Early Diagnosis of Industrial Lead Poisoning. Paper contributed to the Second International Congress for the Study of Industrial Diseases held at Brussels, 1910.
[230]
—Stress has been laid on the wearing of overalls and head coverings in processes giving rise to dust or to splashes of glaze or paint in special rules and regulations in the past. With the improvement that has taken place in exhaust ventilation, they have become less important. Indeed, the aim of all manufacturers and ventilating engineers should be to render processes so free of dust as to make them altogether unnecessary. Increasing knowledge of the insidious manner in which lead dust can arise has shown that from this point of view overalls of the cotton or linen material ordinarily worn constitute a real source of danger. Splashes of glaze dry on them, and at every movement involving rubbing of the surface dust is generated. Some operations almost oblige the worker to press the article worked upon against the chest, so that chance of inhalation of dust from this source alone is considerable. Taking them off creates dust, and after this is done a reprehensible practice exists of either shaking or beating them against a post. In large factories they are usually washed on the premises, and the water in which this is done will become a solution containing some lead in suspension. Hence, even when washed and dried ready for wear, overalls may not be quite free of lead. Apart from the general obligation recognized that an employer should provide and maintain everything necessary to guard against danger contracted in his factory, we think that the risk run by a worker in taking the overall home and washing it there is negligible, and offers advantages in the ultimate cleanliness of the overall over washing on factory premises. Laundresses shaking overalls prior to washing them have been known to contract plumbism.
Where work with the arms is incessant, as in the heading of[231] yarn, overalls are burdensome, and for this reason and the fact that with efficient exhaust ventilation there should be practically no dangerous dust, the regulations for the heading of yarn enjoin provision of them only on written certificate of the Chief Inspector of Factories.
Protection for the clothing, however, where splashing is incidental to the operations, cannot be dispensed with. Hence either the overalls themselves or the front of them should be made of some light ventilated waterproof material, or a waterproof apron worn over overalls of the kind at present in use. Daily sponging would then take the place of washing.
If lead dust be visible on the hair of workers, there must be a defect in the conditions of work to be rectified by other means than cumbering the head. In our opinion, head coverings ought never to be necessary. We cannot think that an attack of plumbism can ever be precipitated by the amount of lead dust inhaled on brushing the hair.
A common provision in regulations for overalls is:
“Overalls shall be provided for all persons employed in lead processes, and shall be washed or renewed every week.
“The occupier shall provide and maintain for the use of all persons employed in lead processes—(a) A cloakroom or other suitable place in which such persons can deposit clothing put off during working hours, and separate and suitable arrangements for the storage of overalls required by regulation; (b) a dining-room, unless all workers leave the factory during meal-hours.
“All persons employed in lead processes shall wear the overalls provided ... and shall deposit such overalls and any clothing put off during working hours in the places provided under the regulations. The overalls shall not be removed by persons employed from the factory or workshop.”
General sense of propriety—and it is on this ground, and not from danger attaching to non-observance, that we press it—suggests that overalls should be kept apart from working clothes, and preferably outside any room in which a lead process is carried on. So long as actual contact between the two sets of garments is prohibited, we do not see objection to the same cloakroom sufficing for both. The best arrangement that we have seen is a room in which each worker has two lockers—one for the storage of overalls, and the other for clothing put off during working hours. This presupposes supervision and effective discipline. We think that all reasonable need in the provision to be made is met by numbered pegs on one side of the room or wide passage for clothing, and on the other pegs correspondingly numbered for the overalls. Means for heating and drying the clothes should not be overlooked (see Fig. 8).
[232]
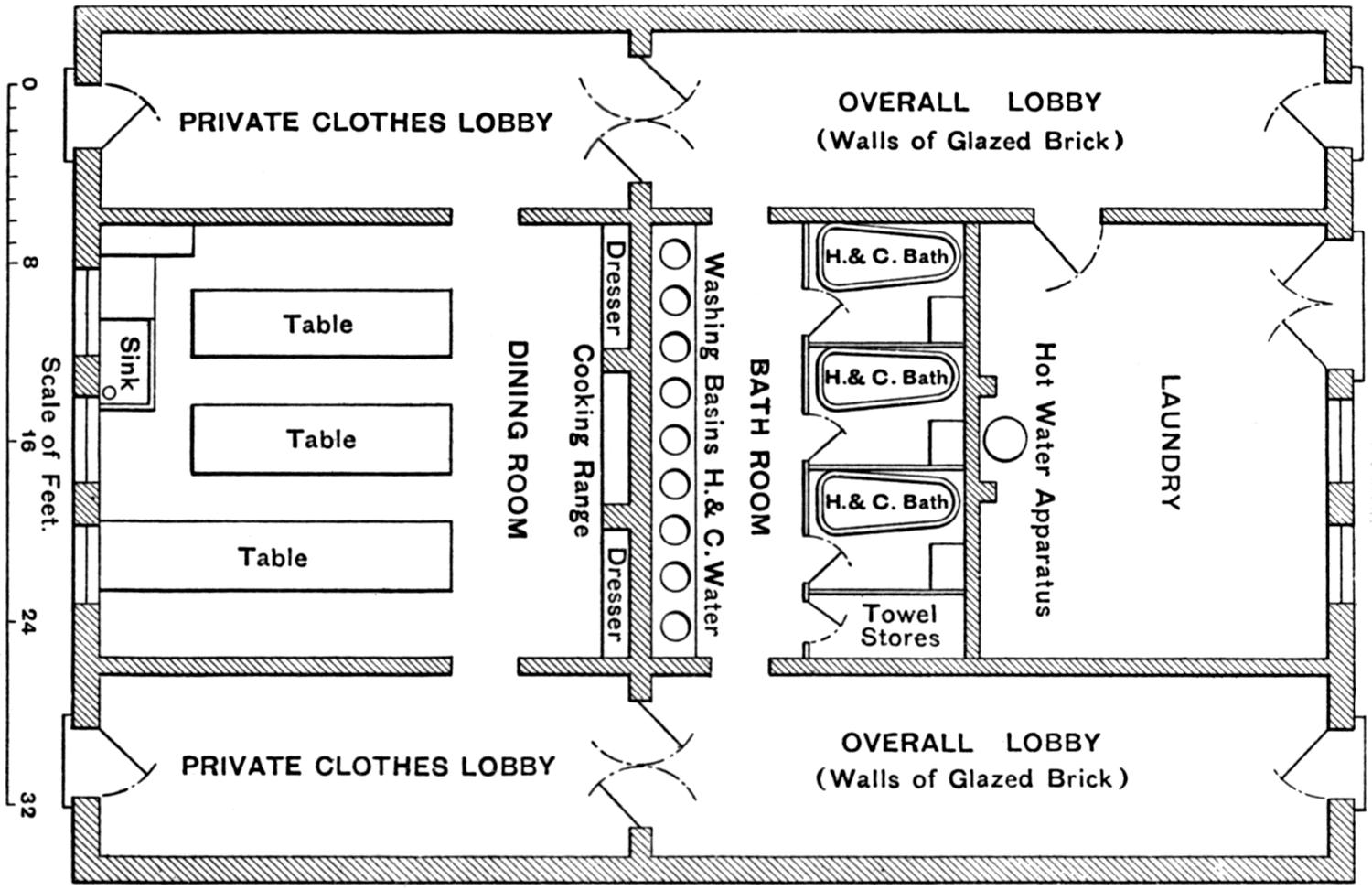
Fig. 8 shows a good arrangement in a white-lead factory, by which, as is best, cloakroom, meal-room, and washing and bath accommodation, are all under one roof. The men on entering hang up their clothes in the private clothes lobby, and pass through the swinging doors to the lobby where the overalls are hung. On leaving the factory and at midday they enter by the door leading into the lobby for storing overalls, and from that pass to the lavatory and bathroom. Having washed and put on their ordinary clothes, they enter the meal-room. The building throughout is lined with white glazed bricks.
[233]

Fig. 8a.—Well Lighted Mess-Room in a Smelting Works.
[234]
—Wherever lead processes are carried on the provision of a mess-room is called for in a part of the factory remote from possible contamination from lead dust. This we hold on general grounds of cleanliness and self-respect, and not because we think the eating of meals in rooms where lead is used would noticeably increase the number of cases. The more conveniently situated in relation to the workrooms the mess-room is, the more will it be used. Set out briefly, the requirements of a mess-room are that it should be—
1. Well ventilated, warmed, and lighted.
2. Not less than 10 feet high, and with floor-space ample for each person likely to occupy it at any one time.
3. Have walls the surface of which is smooth and impervious to a height of at least 4 feet from the ground.
4. Have pigeon-holes or other arrangement in which each person can deposit his food separate from that of others.
5. Have means of warming and cooking food.
6. Be kept properly clean and dry.
No part of a factory becomes so unsightly in the absence of daily cleaning as a mess-room, especially where provision has only to be made for five or six workers.
Reid[1] has suggested the following scale of floor-space per person in mess-rooms:
| 6 persons and under | 10 | ¹⁄₂ | square | feet per | person. | |||
| Over | 6 | and up to | 12 | 7 | ¹⁄₂ | „ | „ | „ |
| „ | 12 | „ | 20 | 6 | „ | „ | „ | |
| „ | 20 | „ | 28 | 5 | ¹⁄₂ | „ | „ | „ |
| „ | 28 | and any number | 5 | „ | „ | „ | ||
In a factory well known to us for manufacture of white lead there is a restaurant originally started in connection with the sick club of the factory. For fivepence the workman obtains a hot meal of meat, bread, and vegetables. Any profits go to the sick fund. Since this has been started improvement in the physical condition of the men has been marked; several cases of anæmia and malnutrition have entirely cleared up. No workman, as has already been emphasized, should commence work in a lead factory unless he has had a good meal—that is, unless there is some food at least in the stomach—particularly albuminous food, such as milk, cocoa made with milk, or café au lait. The most suitable foods generally for lead-workers are those containing proteids—meat, eggs, milk, cheese, and fatty foods. Acids—vinegar, pickles, and the like—are especially to be avoided.
[235]
Section 75 (2) of the Factory and Workshop Act, 1901, requires that, where lead or other poisonous substance is used so as to give rise to dust or fumes, meal-room accommodation shall be provided. The question, as a rule, is easily decided, but there are border-line cases where doubt may arise, as, for example, in letterpress printing factories in regard to dust, and in soldering operations in regard to fume. Operations in the composing-room undoubtedly give rise to dust, and in stereotyping and casting the débris trodden underfoot causes dust to rise. In linotype rooms, however, in the present state of knowledge, a difficult burden of proof would rest on the person who sought to show that dust or fumes were present to such an extent as to justify action under the section. And the same view holds, in our opinion, in regard to soldering.
—The usual requirement for this in nearly all regulations is:
The occupier should provide and maintain in good repair for all persons employed in lead processes—
(1) Suitable lavatory accommodation, including at least one lavatory basin for every five such persons, fitted with a waste pipe, or placed in a trough having a waste pipe, and having a constant supply of hot and cold water laid on; or alternatively troughs of enamel or similar smooth impervious material, fitted with waste pipes without plugs, and having a constant supply of warm water laid on, and affording a length of at least two feet of trough for every five such persons.
(2) Soap, nail-brushes, and a sufficient supply of clean towels, renewed daily.
Discipline, and responsibility placed on some one person to see to the cleanliness of the lavatory appliances and adequate supply of the necessary means for washing, can alone insure proper use of them by the persons employed. The workman has so narrow a margin of time in which to get his breakfast and dinner that he cannot be expected to wash if facilities for doing so fail. The alternative of an enamelled iron trough with jets of warmed water is in our experience much the most satisfactory installation where the number employed is more than five or six. Provision of soft soap or of soap in the form of powder, and nail-brushes nailed to the table, hinder peculation. Wooden stands for holding wash-basins present almost invariably a most uninviting appearance unless covered with sheet lead.
Well-equipped wash-hand basins with hot and cold water laid on close to the work-place are sometimes provided in addition to the lavatory proper. If looked after they are valued and used, but if not they become converted into receptacles for oddments.
[236]
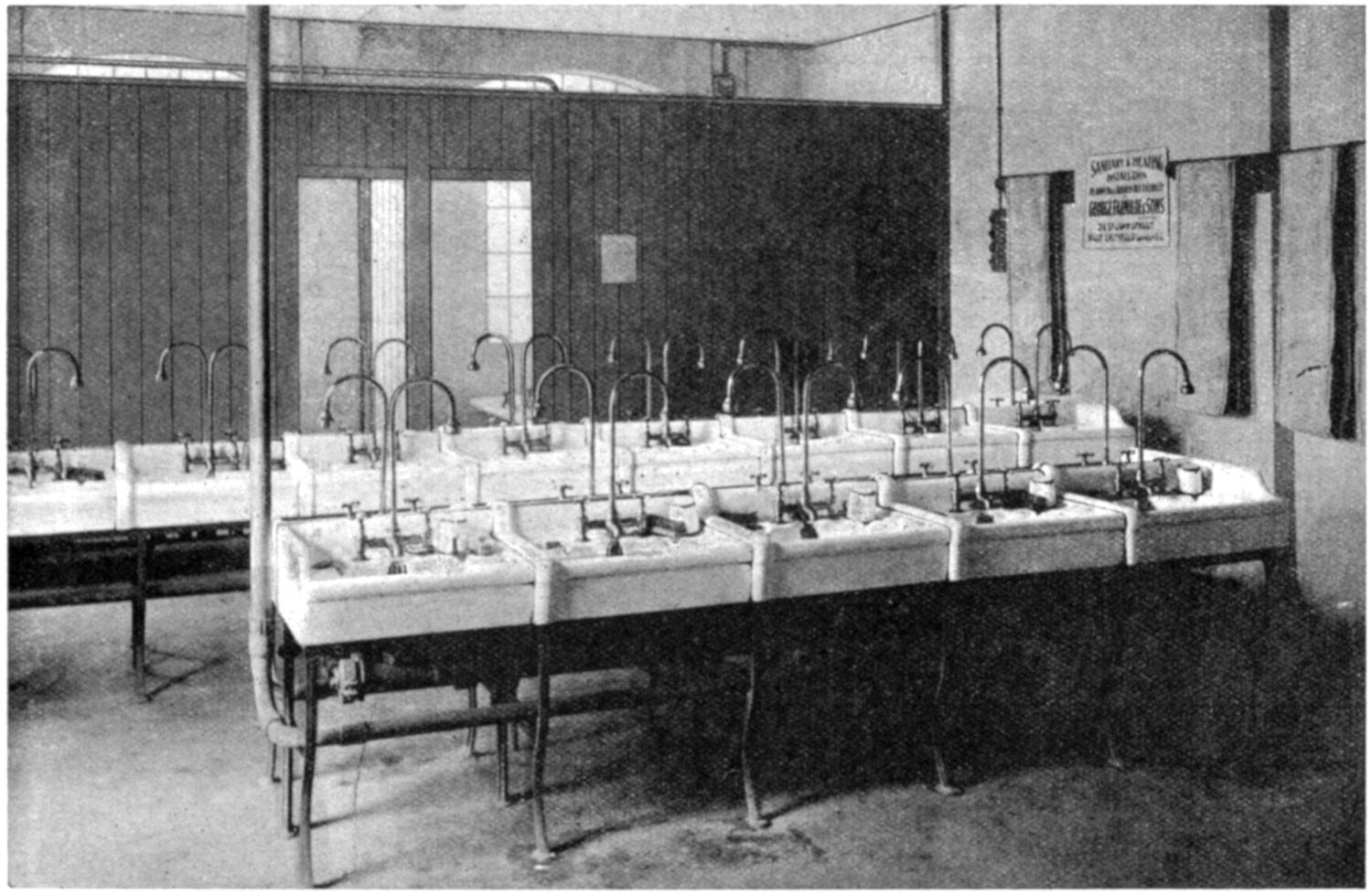
Fig. 9.—Good Washing and Bath Accommodation in a Lead Smelting Works.
[237]
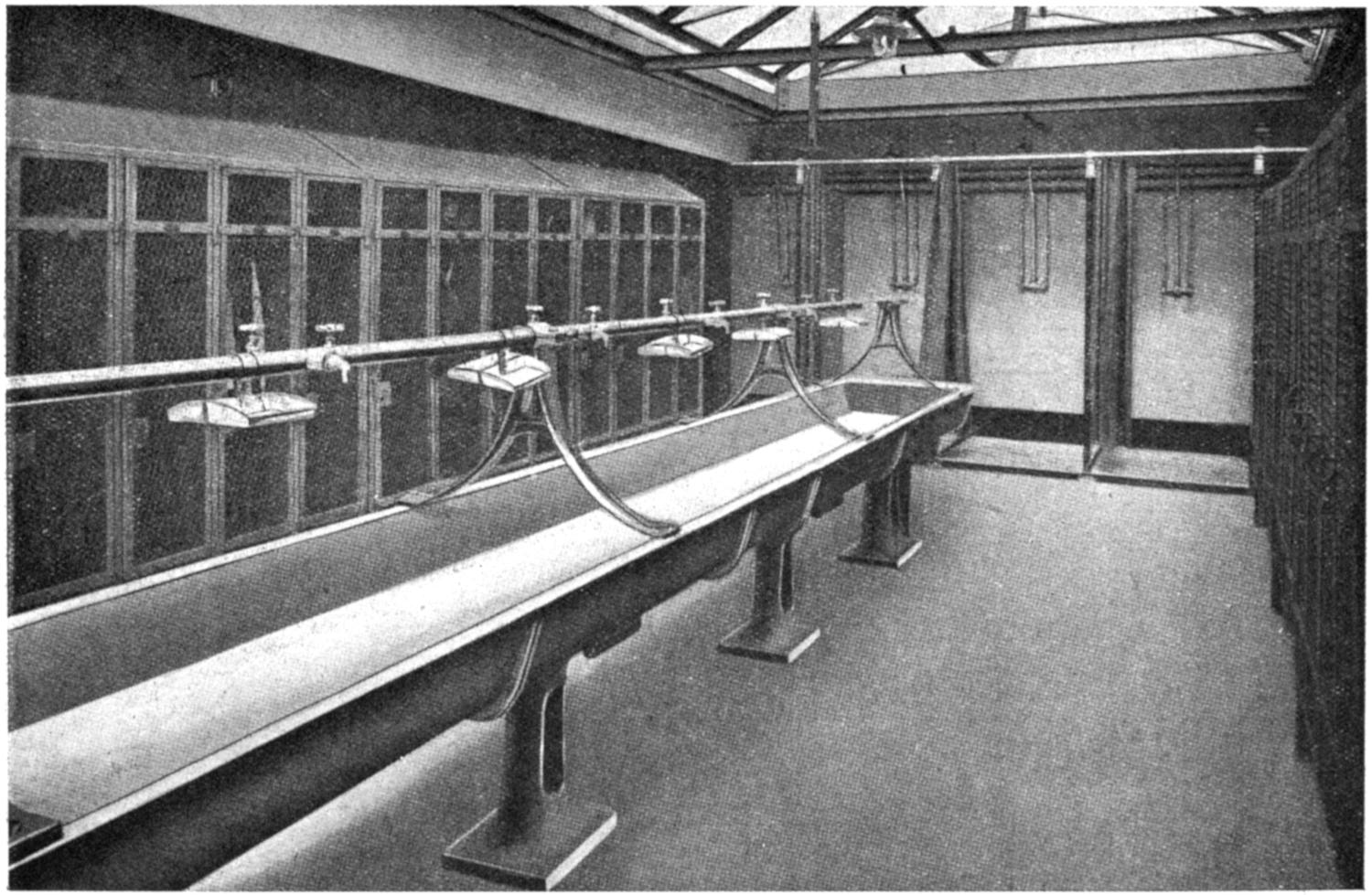
Fig. 10.—Washing Trough, Douche Baths, and Clothes Cupboards.
Type common on the Continent.
[238]
Roller towels should be at least 15 square feet in area for every three persons employed, and renewed daily.
The merits of soluble sulphides in “akremnin” soap have been advanced on the ground that the lead is converted into the insoluble black sulphide, which is thereby made visible to the eye, and the sight of which will, it is hoped, still further stimulate the desire to wash. Probably such lead as remains on the hands after well washing with soap is so closely adherent to the skin as to render risk of contamination of the food in this way negligible. Dr. Robertson, Chemist of the Royal Gunpowder Factory at Waltham Abbey, has found that results as good as those to be obtained from use of akremnin soap (and without any discoloration of the skin) are obtained from use of a solution made up as follows: Sal ammoniac added to saturation to a solution containing 8·5 c.c. of commercial hydrochloric acid (specific gravity, 1·15) per 100 c.c. of water. The procedure adopted at Waltham Abbey is—(1) Usual washing with soap, water, and a nail-brush; (2) scrubbing with the special solution by means of a nail-brush dipped in it; (3) rinsing in water; and (4) washing with soap and water.
Sommerfeld[2], from his wide experience, considers that pumice-soap, with the addition of turpentine, is the best cleansing material lead-workers can use.
—Provision of bath accommodation is required under the special rules for white-lead factories and the regulations for electric accumulators. In several factories, however, baths are provided for the use of the workers. They naturally make for improvement in general health, but we cannot regard provision of them, any more than cloakrooms and mess-rooms, as likely to influence materially incidence of lead poisoning in a factory. The three requisites of (1) convenience and quickness, (2) comfort, and (3) regular use, can best be secured by the use of shower baths in place of slipper baths. The advantage they have are—(1) Initial cost of installation is less; (2) economy in space; (3) economy in water; (4) economy in time required for bathing; (5) no worker comes into contact with water used by another; (6) cleansing must be from the head downwards. Danger of scalding can be absolutely excluded by use of valve levers, so placed in relation to one another that steam cannot be turned on[239] until the cold-water valve has been opened, and the heater can be so arranged that the water is not raised above a definite temperature.
—Lead processes should, as far as possible, be carried on in such a way that there is aerial disconnection of one process from another. Separate workrooms, although desirable unless very small, may not always be possible, but by exhaust ventilation processes may be arranged so that dust is not a source of danger to persons working elsewhere. Instances could be given where, in paint and colour works, persons employed in the grinding of earth colours, in which no lead entered, succumbed to attacks of plumbism from contiguity of the plant with that for grinding lead colours. Threading up of castor runners on a wire previous to dipping used to be done, prior to 1901, in the dipping-house itself, with the result that some severe cases of poisoning occurred. By the rules of 1901 the process was required to be done in a place separate from any place where dipping (or other scheduled process) was carried on, and very few cases have occurred since. And if exhaust ventilation is relied on, Duckering’s experiments (p. 206) show the attention to detail necessary to prevent air charged with lead dust being drawn to the spot by the fan. The need for separation of processes becomes less in proportion as the cubic space of the room increases, and dust and fumes are locally removed.
—The same considerations as have been stated above govern this point also. Wherever lead dust or fumes arise, whether exhaust ventilation is applied or not, persons of either sex under eighteen years of age are probably rather more susceptible to attack by reason of natural failure to appreciate the risk run. When periodical medical examination in addition to exhaust ventilation has been adopted, the age limit can safely be reduced to sixteen. Where handling only of metallic lead and ordinary soldering with an iron are done, risk of contracting plumbism is so remote that an age limit may be unnecessary.
—Wherever walls are likely to be splashed, as in dipping rooms and enamelling workshops, the inner surface of the walls should be of glazed bricks or tiles or enamelled plates, any of which help also in increasing the light necessary in such rooms. If, on account of the cost, such perfectly smooth material is impracticable, the walls should be painted with oil colour, which will enable them periodically to be wet-cleansed. Dusting the walls is to be deprecated. Hence accumulations of dust and[240] débris on ledges, rafters, beams, roofs, etc., should, in our opinion, only very occasionally be cleaned down. We consider that the risk from one big cleaning down is less than that from repeated small ones. (See p. 218).
—These should be smooth and impervious. Wood flooring can hardly be regarded as coming within this category; it is unsuitable where lead processes are carried on. Preference must be given to floors of concrete and the like material, which can be washed down easily rather than swept. Frequently the dust raised in sweeping the floor has determined an attack of lead poisoning. No objection can be raised to wooden grids and iron roll matting placed on the concrete floor for the workers to stand on. Cold feet and transference of lead dust by the boots are lessened by their use. Where traffic is heavy, as is often the case in lead works, vibration is set up with wooden floors, and dust disseminated. In smelting works cast-iron plates are serviceable as flooring.
—Too much stress cannot be laid on this point. To insist upon it, however, is useless unless the occupier has installed the necessary exhaust ventilation, and equipped his factory with the other essentials for enabling the workman to guard against dust and free his hands of adhering lead, in whatever form. When this has been done, then the worker himself can do something.
Often he creates dust unnecessarily. In shovelling out of a cask with spade or trowel, in order to get out the last trace of the material to be transferred, he generally gives it a knock, which causes dust to fly so sharply as to escape the exhaust; or he removes the implement quickly from the exhaust, before it has been completely emptied and while dust is still being given off. He will use a brush to clean his bench in preference to cleaning it by a wet method. The printer may hold type between his teeth. Many workers bite their nails. Moustache and beard may be fingered, and because of this workers may even be advised to be clean-shaven. They may chew or smoke constantly while at work, and will frequently eat with unwashed hands.
Notices enjoining care and cleanliness may be, and often are, hung up in the workrooms, or a leaflet, such as the one here printed, is handed to the worker on his first examination by the surgeon. Nothing, however, can take the place of actual verbal instruction from occupier, foreman, and fellow-worker impressed with the importance of the matter.
[241]
Unless great care is taken, work in lead and lead compounds is injurious to health, because the lead enters the system and causes lead poisoning.
Danger is greatest from breathing leady dust or fume, but eating with unwashed hands, and biting nails or putting such things as sweets or pipes into the mouth while the fingers are soiled with lead, all help to cause poisoning.
Many workers in lead have a blue line round the edge of the gums, but the first actual symptoms of the injurious action of lead on the system are costiveness, colicky pains in the stomach, headache, and marked paleness. Occasionally headache is associated with epileptic fits, a very serious condition, which may be followed by loss of sight. “Wrist-drop,” loss of power in the muscles moving the fingers and wrist, results sometimes from lead poisoning, and may cause permanent disablement from work. It does not usually come on until after a person has worked in lead for some years.
Lead, even if absorbed in small quantities, has a tendency to remain in the system, and if care be not taken it will go on accumulating, so that in time the health may become permanently damaged, even without any definite attack recognized as lead poisoning.
Girls and boys, because they are not so likely to observe the necessary precautions as grown-up people, should be closely watched. Women should be especially careful, as the injurious effect of lead in them may seriously interfere with the healthiness of their children.
As lead does not enter the system through the pores of the skin, it can in great measure be avoided by—
1. Taking special care to avoid raising dust. It is to the interest of everyone to see that ventilating arrangements are in order for carrying dust away at the point where it is produced.
Any little cloud constantly made at work is sure, if breathed, to set up lead poisoning. Where lead colours are used wet, danger arises from the splashing of the material and its subsequent drying into dust.
2. Paying scrupulous attention to cleanliness of the hands, face, teeth, and clothing. The hands and nails should always be cleaned with soap and nail-brush before food is eaten, and it is a wise practice also to wash out the mouth. The teeth should be brushed at least once a day, preferably before the evening meal.
3. Never commencing work on an empty stomach. Food containing fat, such as bacon and milk, is suitable.
Overall suits, if worn, should never be shaken to rid them of dust. They require washing at least once a week.
Aperient medicine, such as Epsom salts (one or two teaspoonfuls in water), can be taken once or twice a week with advantage by lead-workers.
Experience shows that the habits and home life of the workers influence their liability to lead poisoning. Intemperate persons are the first to fall victims. Those who begin work on an empty stomach incur additional risk by so doing.
Carefulness while at work, and cleanliness, offer the best means of escaping attacks of lead poisoning.
Those who work in lead should keep in mind every hour of every working day the importance of not breathing lead dust and not carrying lead to the mouth in any way.
Medical advice should at once be obtained if signs of lead poisoning present themselves.
[1] George Reid: Memorandum on Mess-room Accommodation: Appendix XXV. of the Potteries Committee’s Report, vol. ii., 1910. Cd. 5278.
[2] Th. Sommerfeld: Die Bekämpfung der Bleigefahr, edited by Leymann, p. 76.
[242]
Lead smelting—Red and orange lead and litharge—Letterpress printing—File-cutting—File-hardening—Tinning of metals—Plumbing and soldering—Brass.
—Lead poisoning very rarely occurs in lead mining in Europe, as galena (sulphide of lead), the principal ore in which the metal is found, is insoluble. Galena always, and other lead ores very often, contain a small proportion of silver, ranging from 0·001 to 1 per cent., and at times traces of gold. Owing to the great affinity of lead for silver, lead smelting is necessarily a process preliminary to the extraction of silver and gold from it[1].
Lead ores, drosses, etc., on arrival at the factory, are, after sampling, deposited in bins or heaps (often in the open air), and watered to prevent dust. All ores may, and refractory ores (containing over 4 per cent. silica) and dross must, be smelted in a blast furnace by aid of coke. The bulk of the charge in a blast furnace may consist of more or less complex ores of the precious metals, especially silver.
When galena is treated in a blast furnace, preliminary roasting is indispensable, and in many smelting works its treatment takes place in a reverberatory or open-hearth furnace, and not in a blast furnace.
The three principal methods applicable to extraction of lead from ores are—(1) The roast and reaction method; (2) the roast and reduction method; and (3) the precipitation process.
By the roast and reaction method a part of the galena is first converted into oxide and sulphate of lead with access of air. Subsequently, on shutting off the air-supply and increasing the temperature, a reaction takes place. The sulphur in the unchanged sulphide combines with the oxygen of the oxide and[243] sulphate to form sulphur dioxide, which is carried away by the draught into the bricked flue, leaving metallic lead behind. The process is carried on in a reverberatory or open-hearth furnace.
In the roast and reduction method the first portion of the process is carried out in a reverberatory furnace, the galena being roasted pretty completely to lead oxide and sulphate, which are then—usually in a blast furnace—reduced to the metallic state with coke and other reducing agents, such as iron.
By the precipitation process galena was decomposed at a high temperature by means of metallic iron, forming a mixture of iron and lead sulphide. This method was only applicable to rich lead ores, and is now given up.
The three methods are hardly ever independent of one another, as the rich slag or residues, for instance, which are obtained by the first method are retreated by the second, and the second is, as has been stated, almost always combined with the first.
On tapping the blast or reverberatory furnace, the lead is drawn off into a lead well or sump, from which, when cool, it is ladled into moulds, while the slag is run into movable metal pots or along specially-prepared channels. The slag run off from the reverberatory furnace contains much lead locked up as silicate, which requires to be retreated, usually in the blast furnace. During the roasting process much raking of the material is necessary. The slag from the blast furnace should contain less than 1 per cent. of lead.
On the Continent and in America, the Huntingdon-Heberlein process has been extensively adopted, with lessened incidence of poisoning, the result of mechanical methods of working, obviating hand labour, and the low temperature (diminishing risk from lead fume) at which the roasting is carried on. In this process the crushed ore is desulphurized by first mixing with lime and heating in presence of air in a revolving furnace, provided with automatic rabble, at moderate temperature (about 700° C.). Subsequently the roasted material is conveyed from closed bins, into which it falls automatically, by dust-proof elevators to a converter, in which atmospheric air at slight pressure is forced through it. The agglomerated mass so formed, when tipped out of the converter (in doing which there is risk from dust), is well damped, broken by hand, and charged with coke in the usual way into the blast furnace.
In some lead-smelting works the material arrives on the[244] premises in the form of ingots of base bullion—i.e., impure lead rich in silver—the product of previous smelting of the ore where it is mined in Australia or Spain. And one of the main objects of the blast-furnace smelting of galena in the factory is to produce a base bullion rich in precious metals. The lead so obtained requires further softening or refining to get rid of copper, antimony, arsenic, and tin. This is effected in a reverberatory furnace, first at a low temperature to allow of formation of furnace dross, which is removed through the working doors, and secondly with increase of heat and access of air to oxidize, in the order named, the tin, arsenic, and antimony. Finally the lead is tapped into kettles or pots. If free from silver, such lead, when poured into moulds, is ready for the market; but if rich in silver, it is treated for the recovery of that metal either by (a) Pattinson’s process, depending on the higher temperature of crystallization of lead than of an alloy of lead and silver, which enables a separation of one from the other to be made by a process of ladling the crystalline from the liquid portion; or, much more commonly, by (b) Parkes’s process, depending on the formation, on addition of zinc to a pot of molten lead, of crusts consisting of an alloy of silver, lead, and zinc. The crusts obtained in the latter process, after cooling, are broken up, placed in a crucible, and the zinc driven off at a temperature of 1,000° C. in a dezincing Faber du Faur retort. The rich bullion, retained either in the last kettle by the Pattinson process, or remaining in the crucible after dezincing, next undergoes cupellation—i.e., exposure to a blast of air in a furnace. The lead is oxidized into litharge, which drops into a receptacle below the furnace, leaving the silver behind. In all lead-smelting works the draught from the furnace carries much dust of ore and fuel, and fume, consisting of sulphide, sulphate, and oxides of lead, into the flues. The dust is easily collected in dust chambers, but the fume requires ducts of great length—sometimes a mile or more—in which to deposit.
—The risk from dust in general labouring work, in depositing the ores in bins, in removing them to, and charging them into, the furnace, can only be controlled by watering, preferably by a spray. From the blast furnace lead fume and carbon monoxide may escape at the point where charging is done, if there is back pressure from blockage in the flues, or if the furnace blast is not working perfectly. In tapping the lead and in manipulations such as charging,[245] drossing, and skimming, conducted through the doors of furnaces of all descriptions, hoods, extending at the sides down to the floor level, require to be arranged over the working doors, and connected either with ducts passing vertically through the roof or directly with the exhaust created in the furnace or flue itself. Dross and skimmings removed through the working doors should be received into iron trolleys capable of being covered, and not be allowed to fall on to the floors, to be shovelled up later on to barrows. Before such dross or slag from reverberatory furnaces is broken up for further treatment it should be well watered.
Lead absorption among the men actually employed in the Pattinson and Parkes’s processes is comparatively rare, as the temperature of the molten metal does not exceed 450° to 500° C. When, however, the zinc-silver-lead and gold alloy is removed for treatment in special furnaces for distillation off of the zinc, prior to cupellation, the lead from the Parkes’s pot, now free from silver, but containing traces of zinc, antimony, and other impurities, is run in some works into what are termed “market pots” for a final refining. Air and steam are blown through to oxidize the impurities. The pot is skimmed twice, the first dross containing antimony, etc., and the second a fine dust consisting of lead (60 per cent.) and zinc. The risk of poisoning at this point is considerable, although an exhaust fan connects up the cover of the pot with a cyclone separator, to carry away the fume when the steam is blown through. In other works this dezincing is done in a refining furnace, the material being then in a slaggy state, thus hindering development of fumes. After the condensation of the zinc in the distillation of the silver-lead and zinc crust the cover of the pot is raised, and the remaining metal, containing 80 per cent. of lead at a temperature of about 2,000° F., is ladled out into moulds for treatment in the cupelling furnace. The temperature at which this ladling operation has to be done makes the work impossible for those unaccustomed to it. Exhaust ventilation in the operation of emptying the pot, and cutting off the heat by a water-cooled jacket, suggest themselves as means to combat the undoubted risk.
In cupellation the temperature is high (about 2,000° C.), and fume will escape from the working door and from the opening where the rich lead is fed into the furnace. The danger here is sufficiently recognized by hoods and ducts placed in front of[246] the furnace, but the draught, unless the ducts are connected up with a high-pressure fan, may prove inadequate to carry away all the fume.
Flue-cleaning, carried out usually at quarterly or half-yearly periods, is dusty work, as much of the dust is in so fine a state of division as to repel contact with water.
Smelting of other metals when the ores contain appreciable amounts of lead is equally productive of plumbism. Thus, in the year 1901 fourteen cases were reported from an iron works for the manufacture of spiegeleisen, the ore (now no longer used) coming from Greece[2]. In previous years it would appear to have been even greater. A remarkable feature of all the reported cases from this factory was that the form assumed was colic, and never paralysis. The poisoning was due to vaporization of the molten lead by the very high temperature to which it was raised as the molten iron flowed out of the furnace on tapping. The danger from fume was limited to the first few feet of the channel, as the heavier molten lead gravitated down between loose brickwork into a pit. Dust collected above the point where the furnace was tapped contained 39·77 per cent. of lead monoxide, and the flue dust 4·22 per cent.[3]. A flannel respirator worn once only by one of the furnace men contained lead equal to 16 milligrammes of lead monoxide. In 1906 three cases were reported in the extraction of copper. The persons affected were employed in charging ore into the cupola[4].
Heavy incidence of poisoning (twelve cases in two months) in a smelting works (now closed) led to examination of sixteen men. The gums of only one man were free of a blue line—in most it was particularly dense—eight were anæmic, one had paralysis of the wrists, and five others weakness. Analysis of the air was made at different points in the factory by the chemist of the works, G. D. Cowan, with the following results:
The samples from the cupola were taken from inside the hood (about 5 feet above the men’s heads). The gas was filtered through cotton-wool, so that all solid particles were retained, and the remaining gas was treated separately. The solid particles will be called “dust,” and the gas, after filtration, “fume.”
The cupola samples on being examined gave—
| Dust, | first sample | 0·08152 | grain of | lead per | cubic foot. | ||
| „ | second sample | 0·07297 | „ | „ | „ | ||
| Fume, | first sample | - | 0·00526 | „ | „ | „ | |
| „ | second sample | ||||||
The samples from the lead well were taken 12 inches above the molten metal at the end of the lead siphon, and gave the following results:
| Dust | 0·05653 | grain per cubic foot. |
| Fume | Nil. |
[247]
The briquetting machine samples were taken from the platform where all the ore and fluxes are mixed before briquetting.
The results obtained here were as follows:
| Dust | 0·95715 | grain of | lead per | cubic foot. |
| Fume, or fine dust that passed through filter | 0·01314 | „ | „ | „ |
The reason for these high results was owing to dust raised when waggons of ore were tipped prior to mixing.
Assuming that 20 cubic inches of air pass in and out of the lungs at each respiration, a man in eight hours would inhale and exhale 94·4 cubic feet. This amount of air, inhaled at the position in the cupola where the sample was taken, would contain 7·3818 grains of lead; at the lead well, 5·3064 grains; and at the briquetting machines, 91·5953 grains. Although the condition of the air where the men actually worked must have contained much less than these amounts, the analyses quite serve to explain the heavy incidence.
Collis[5] quotes the following analysis of dust and fumes from Hofman’s “Metallurgy of Lead.”
Lead Smelting: Analyses of Dust and Fumes (from Hofman’s “Metallurgy of Lead”).
| Material Analysed. |
Percentage of— | |||||||
|---|---|---|---|---|---|---|---|---|
| Arsenic. | Arsenious Oxide. |
Lead. | Lead Monoxide. |
Lead Sulphate. |
||||
| (1) | (2) | (3) | (4) | (5) | (6) | |||
| All dust collected in ten years, average | — | — | 25·6 | — | — | |||
| Dust from— | ||||||||
| Downcomers of eleven blast furnaces | — | — | 47·5 | — | — | |||
| Roof of blast-furnace building | — | — | 27·1 | — | — | |||
| Fumes from— | ||||||||
| Slag pot while boiling | — | 4·8 | — | 41·0 | 26·2 | |||
| Reverberatory settling furnace | 2·3 | — | — | 31·0 | — | |||
| Flue dust— | ||||||||
| Friedrichshütte, Silesia | — | — | — | 62·8 | — | |||
| Freiberg, Saxony | - | A | 7·5 | — | 26·2 | — | — | |
| B | 37·5 | — | 21·3 | — | — | |||
| C | 46·4 | — | 16·2 | — | — | |||
| Pribram, Bohemia | — | 1·0 | — | 45·5 | — | |||
Collis[6] estimated the attack rate in lead-smelting works at 30, and in spelter works at 10, per 1,000 per annum. In one factory he found it 80 per 1,000, and in a spelter works five cases occurred in a few months among seven workers.
[248]
The distribution of the reported cases from year to year was as follows:
| Process. | 1900. | 1901. | 1902. | 1903. | 1904. | 1905. | 1906. | 1907. | 1908. | 1909. | 1910. | 1911. | Total. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lead smelting | 21 | 26 | 13 | 13 | 7 | 10 | 16 | 21 | 31 | 28 | 21 | 33 | 240 |
| Desilverizing | 1 | 3 | 9 | 10 | 16 | 6 | 9 | 4 | 3 | 6 | — | 3 | 70 |
| Spelter | 5 | 11 | 3 | 4 | 4 | 5 | 9 | 2 | 31 | 25 | 12 | 11 | 122 |
| Other (copper, iron, etc.) | 7 | 14 | 3 | 10 | 6 | 3 | 4 | 1 | 5 | 7 | 1 | 1 | 62 |
| 34 | 54 | 28 | 37 | 33 | 24 | 38 | 28 | 70 | 66 | 34 | 48 | 494 |
—Lead is present in zinc ores in a proportion of from 1 to 10 per cent. (usually 3 per cent.). Despite this small proportion, incidence of chronic plumbism among those engaged in the manufacture is high, as in the present state of knowledge the lead fume given off in distillation of the zinc cannot be efficiently removed. Blende (zinc sulphide) is first calcined, and the residue, after mixture with calamine (zinc ashes) and anthracite, forms the charge for the furnace. The retorts are arranged in long rows one above the other, and frequently back to back in the furnace, so that there may be 250 or more to each furnace, and of the furnaces there may be several in a shed. Attached to the retort is a fireclay receptacle (condenser) into which the zinc distils, and an iron nozzle (prolong) to prevent oxidation in the condenser. While distillation goes on the carbonic oxide gas evolved burns brightly, tinged with the greenish-white colour imparted by the zinc. The products of combustion, with traces of lead fume from the hundreds of prolongs, are discharged into the atmosphere of the sheds, where temperature is high. The latest design of prolongs, however, has an exit at which the products of combustion escape near the furnace, so that the greater portion pass up into the ventilating hoods. Periodically—three times to each charge—the workman removes the prolong, ladles out such zinc as has condensed, and pours it into moulds. Finally, when distillation is completed, the contents of the retorts are raked out, and it is in the fuming hot residues so deposited on the floors that much of the danger arises. In distilling furnaces of modern[249] design the hot residues fall through openings in the window of the furnaces into “pockets,” in which they cool off considerably before they are drawn out into iron skips. In another form of furnace used in the manufacture of spelter (Silesian), the workman after charging can leave the furnace until the time for tapping arrives. The two operations involve work for six hours a day only.
—During distillation the detrimental effect of a current of air (formation of zinc oxide) on the zinc is an obstacle to the removal of the fume by exhaust ventilation locally applied over the prolongs of the condensers. Exhaust ventilation of a kind can, however, be arranged, except under unfavourable weather conditions, by erecting hoods of material such as galvanized iron right across the roof of the shed over, and parallel with, the furnaces, up which the heated current of air from the furnaces travels. Lofty, roomy sheds assist materially in the escape of the fumes. Various forms of modification in the condensers, designed to lessen escape of fume, and so recover more zinc, are being tried.
Samples of fume condensed as a grey powder, and collected by Collis from different kinds of prolongs, showed 1·3 to 2·7 per cent.[7] of metallic lead respectively, and a sample of dust deposited from material containing 10 per cent. of lead, 3·25 per cent.[8].
—These processes are frequently carried on as part of lead-smelting works. Red lead is produced by oxidation, first, of metallic pig-lead, in a reverberatory furnace at dull red heat, into massicot (yellow monoxide). During the process the material is constantly raked. The massicot is withdrawn from the furnace, and subsequently, after drying and sieving, is again subjected to similar treatment at slightly lower temperature. Orange lead is made by treating white lead in the manner described.
During the ten years 1900-1909 the number of reported cases from the manufacture of red lead was 108, of which 47 were attributed to work at the furnaces, 43 to packing and sieving, and 16 occurred among general labourers, part of whose duty it was to sweep up the floors. Collis estimates the attack rate in the five years 1905-1909, in a certain number of factories employing 171 persons, at 50 per 1,000. Reference to the table on p. 48 shows that the proportion of those suffering from encephalopathy[250] is higher than in any other industry—an observation previously noted by Layet[9].
—Danger is practically limited to escape of dust in (1) raking the charge out from the hearth on to iron trolleys, (2) sieving, and (3) packing. In all these operations exhaust ventilation is essential, and for sieving and packing the installation requires to be designed with especial care, so as to be able to keep within the sphere of the exhaust the spading and shovelling of the material, in very fine state of division, into the cask. Sometimes the material is elevated from pits, and eventually packed by mechanical means into barrels resting on a jolter. Unless the elevators are quite dust-proof, and the collar hermetically seals the connection of the shoot with the barrel, the vibration of the heavy machinery and pressure of air inside the casing will cause dust to escape.
Red lead can be, and is, now made on an extensive scale in such a way that all operations, from commencement with pig-lead to the final packing, are carried out by mechanical means so entirely closed in that the worker does not come into contact with the material. The person who then may be affected is the fitter attending to repairs of the machinery. The pig-lead is melted, stirred, and mixed in a covered-in melting-pot. The massicot which is formed is drawn off by an exhaust into a hopper, from the bottom of which it is fed mechanically on to the floor of the furnace. Mechanical rabbles stir it from the centre to the outside of the furnace floor, from where it is conveyed, under negative pressure, to the hopper of a grinding mill. From here it is again similarly fed into another furnace. The exhaust pipe from this furnace collects the finished product, carrying it mechanically to a hopper which automatically feeds the red lead into casks. Negative pressure throughout prevents escape of dust.
—Pig-lead is placed in a cupellation furnace, and constantly stirred and raked over to cause entire oxidation, and then is either raked out or run out from the furnace hearth into moulds, and allowed to cool in the form of large balls. These balls, of a roughly crystalline nature, are deposited on the floor, where they are exposed to the air. Disintegration is accelerated by breaking up the large fragments by hand. Subsequently the material is placed in a disintegrator for fine division and packed.
—Manufacture of litharge may cause[251] a greater amount of dust than any other process with which we are familiar. The nature of the operations is such that it is impossible at all stages to control this dust. Danger is greatest in the early operations of shovelling up the disintegrated powdery material from the floors into receptacles, and in discharging the contents into the disintegrating machine. The work is heavy, and a respirator is with difficulty worn. A movable hood attached to a flexible duct in connection with an exhaust which could be moved from place to place on the floor suggests itself, but when tried it has not effectively controlled the dust, owing to both the trouble involved and the difficulty of bringing the exhaust near enough to the work. When once the material reaches the disintegrator, exhaust over the hopper, and in connection with the enclosed sifter and grinder and packing machine, can readily be secured. Bins should be provided for the litharge lumps, so as to avoid trampling the powder underfoot, and covered barrows for removing the semi-powdered material. Alternation of employment lessens risk, and should be always arranged. In any new plant the possibility of automatic methods of carrying out the process as far as possible should be considered.
—This industry also is not infrequently carried out on smelting premises. To make lead piping, molten refined lead is run into a cylinder containing an adjustable mandrel in its centre. The cylinder is forced by hydraulic pressure against a hollow ram having an adjustable orifice to form the desired thickness of pipe. In the case of sheet lead the thick plates are gradually reduced to the desired thickness by pressure of heavy steel rollers.
—Little risk attaches to handling the clean sheet lead or drawn lead. Danger is in the early stages. Old oxidized lead piping, lead cisterns, tea lead, old accumulator plates, etc., lie in heaps on the premises. These cannot be handled without generation of dust. When melted and stirred, copious fumes arise, carrying up dust, from which, and from that raised in drossing the surface of the metal, absorption of lead is inevitable unless the melting-pot is fully protected from side-draughts and provided with a hood and duct leading into the main chimney-stack. Doors in front of the hood serve still further to confine the fumes. The skimmings from the pot require to be placed in a receptacle under the hood. Of 109 cases reported in the ten years 1900-1909, operations at the[252] melting-pot accounted for at least 47. We are in agreement with Dixon Mann, who remarks: “Workers in metallic lead do not suffer unless they are frequently in the presence of large quantities of the molten metal, or inhale fine particles of solid lead or its oxide whilst manipulating old metal. Lead, though not usually classed amongst the volatile metals, is capable of volatilization at a high temperature, and in the form of vapour may be taken into the system through the respiratory tract, and also into the stomach. One of the worst cases of chronic lead poisoning I ever saw was that of a man who bought the sheets of lead linings of old tea-chests, and melted them down into pig-lead. He did the work in a small room, without any contrivance for ventilation, and attended to the whole process himself”[10].
—In this industry account has to be taken of contact with—
1. Molten lead in (a) casting the type in different kinds of machines, including the monotype and linotype; (b) in stereotyping; and (c) in recasting into moulds the line or single type after it has been once used, together with débris from the stereo machine and sweepings from the floor.
2. Metallic lead in handling and dressing the type, and subsequent use of it by the compositor. The type metal itself usually consists of—Lead, 75 per cent.; antimony, 23 per cent.; and tin, 2 per cent.
During the ten years 1900-1909, 200 cases were reported—92 compositors, 71 stereotype and linotype operators, and 37 in subsidiary processes, mainly in the casting-room. Thus, apparently, operations involving contact with molten metal are more likely to cause lead poisoning than actual handling.
—In letter founding and in the monotype letter-casting machine the molten metal, heated by a coal fire in the former and Bunsen burner in the latter, at regular intervals fills the matrices at a point where it is cooled by a jet of compressed air, and the formed letter is then mechanically ejected into a receptacle. The temperature of the molten metal has to be carefully regulated, and does not usually rise above 400° to 450° C.—a temperature at which it is extremely doubtful if lead fume can be produced. Sommerfeld[11] states that in 60 cubic metres of air aspirated close to a type-casting machine no trace of lead was found, because vaporization does not take place below 550° C. Such skimming as must occasionally be made of[253] the small surface of molten metal is in a slaggy state, and does not appear to contain much oxide. This is deposited usually in a small box and removed to be remelted once a day. What fume, often of unpleasant odour, is noted is probably due to acroleic acid vapour from the grease and dirt.
The letters having been cast, the type may be rubbed on sandstone or on a file, by which small quantities of metallic dust are given off; set up on setting-boards so that all letters face the same way (work on which female young persons are usually engaged); certain portions of letters undercut so as to make them lie perfectly parallel; dressed, planed, and examined, so as to be of precisely the same height; and finally assorted into founts and packed in the warehouse. In all these operations the fingers necessarily get blackened by contact, and there must be slight dislodgment of metal particles to account for the cases reported.
In the linotype machine, matrices are brought down from the magazine by touching the corresponding letters on the type indicator until they are arranged so as to form the line; a lever then carries them sideways into position, so as to allow the molten metal to flow into the mould and cast the line. Another lever then raises the matrices, which are carried into the magazine again, the slab of metal with the cast line upon it falling into a receptacle. Here, again, danger of lead fume is hardly in question. As the matrices drop down from the magazine, particles of lead which they have gathered when in contact with the metal are detached, and are visible on every linotype machine at this point. The brass cover of the magazine, if not frequently cleaned, soon becomes coated with fine dust. Although lead fume may not be given off, it is none the less necessary to remove the products of combustion from the heating apparatus, in order to prevent constant vitiation of air and to reduce the temperature in the neighbourhood of the machines. Monotype machines give off much heat. Exhaust ventilation by hoods reaching well over the pots, and branch ducts entering the main duct in connection with a fan tangentially, can alone accomplish this satisfactorily. Hoods and ducts leading merely into a shaft running up the side of the building fail to prevent condensation of the water vapour, which in consequence trickles back. Wherever a well-thought-out system of exhaust ventilation has been installed, reduction in temperature and comfort to the operatives has been secured. Temperatures above 65° F. must incommode a linotype operator.
[254]
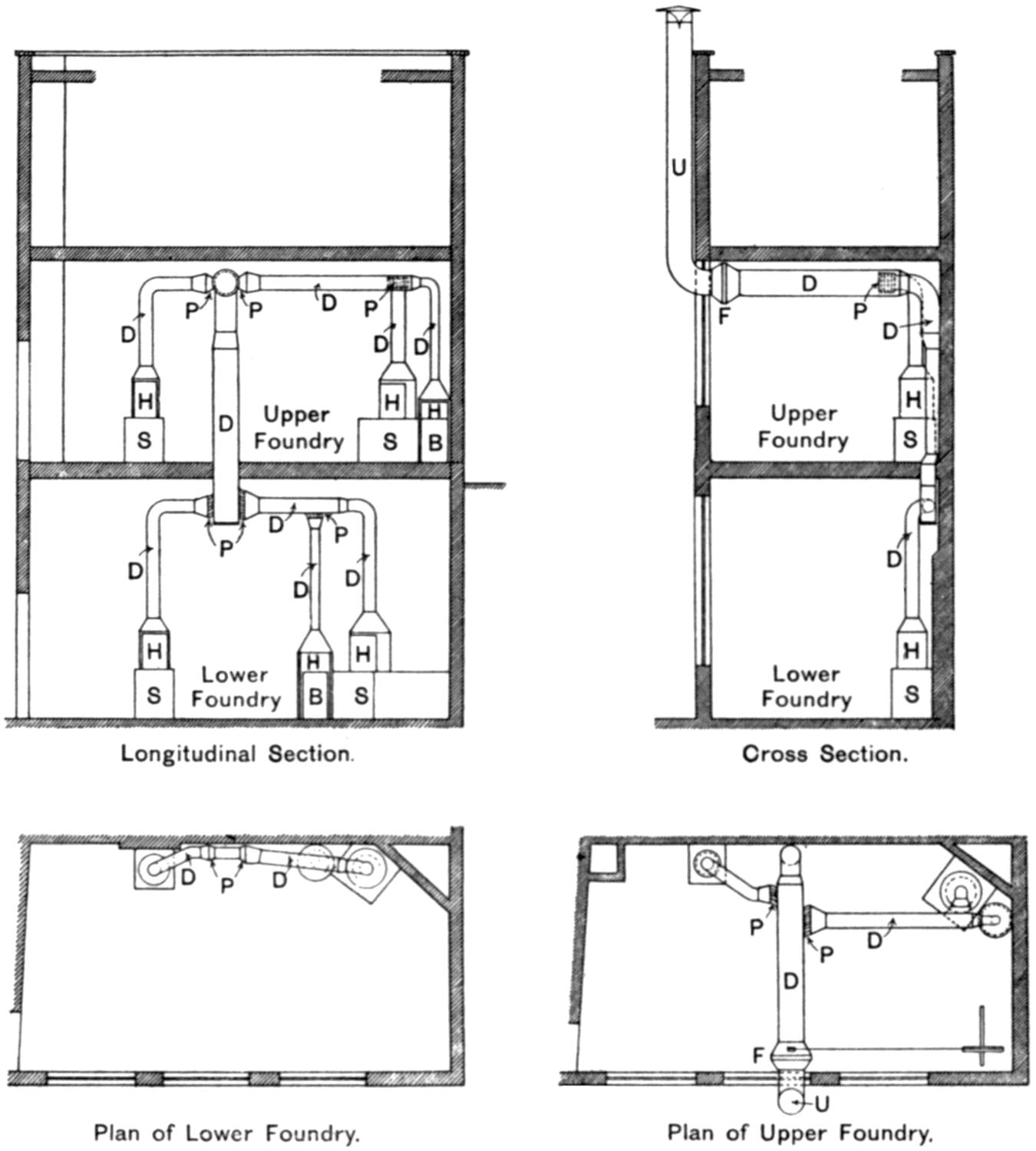
Fig. 11.—Exhaust Ventilation on the Patent “Pentarcomb” Principle applied to Metal Melting-Pots, etc., in Printing Foundry, as installed by the Zephyr Ventilating Company, Bristol.
P, Patent “pentarcomb” for equalizing exhaust; D, main and branch ducts; U, upcast from fan; F, fan; H, hoods over melting-pots and dross drums; S, stereo metal melting-pots; B, boxes or drums for dross.
The illustration shows exhaust ventilation applied to the melting and stereo pots and dross drums in a letterpress printing foundry. The draught over each pot is equalized at the point where the branch ducts join the main duct by insertion of a patent “pentarcomb” grid, which breaks up the columns of air into numerous smaller channels by specially curved metal plates so as to minimize friction. The ducts are graduated, and the exhaust is provided by a volume fan.
[255]
In the foundry the recasting of old used type, etc., is effected, and periodically scraps and sweepings are melted down. These melting-pots should have telescopic hoods so balanced that they can readily be lowered, so as to enclose the bath of molten metal and allow the fume to be drawn by the fan into a duct of such width as to offer no obstacle to escape. A duct too narrow to deal with the great expansion the heated air undergoes is a defect very frequently found. A principal source of danger is the skimming of the melting-pot for sweepings, etc., and deposit of the large amount of dross by the side of the pot. Receptacles for the dross in connection with the exhaust system are imperatively needed.
Exhaust, where practicable, over the often large melting-pot for stereotype casting is desirable, in order to secure a reasonable temperature. Here there is the danger of splashing of molten metal, which is subsequently trodden underfoot.
—The letters are distributed in the small compartments of the type cases. From attrition dust may lie thick in the compartments, and when at work there is always tendency for small quantities of this dust to be dispersed. While this is the principal source of poisoning, inasmuch as dust containing lead must adhere to the fingers, lead may thus enter the system with food or when smoking. It is quite as easy, also, to believe that lead poisoning may result from solvent action of the blood and tissue fluids on small spicules of lead type which penetrate the skin as to credit the well-substantiated cases of plumbism ensuing on retention of bullets or shot in the body. Compositors sometimes contract the habit of holding type between their teeth.
The old dangerous method of blowing out the dust on the staircase by means of a bellows should before long be entirely supplanted by use of suction bellows or use of printers’ case dust-extractors. In the Clements apparatus the cases are placed on a shelf, which is made to oscillate; air is forced into the compartments from numerous jets, so as to raise the dust, which is removed by suction and collected. The cases are thus cleaned with great saving of time in the composing-room itself, and without contamination of the general atmosphere by dust.
The dust removed from a composing box by a vacuum cleaner[256] was found in the Government laboratory to contain 9·8 per cent. of metallic lead, and that collected from the top of the magazine of a linotype machine 8·18 per cent.
Regulations issued in 1911 in Austria require, among other things—(1) Melting-pots, and, so far as is practicable, linotype pots also, to be provided with hoods and ducts to carry the fumes to the outside air or into a chimney; (2) the type cases to fit either close on to the floor or with a sufficient space below the lowest drawer to enable the floor underneath to be easily cleaned; (3) the interior of all compositors’ boxes to be cleaned at least once every three months—if possible, by means of a vacuum suction apparatus; and (4) quarterly periodical medical examination to be made of persons employed in casting, stereotyping, linotyping, assorting type, and composing.
Sommerfeld[12] believes that in Berlin 1·07 per cent. of the compositors suffer from lead poisoning every year, and that 2·5 per cent. of all the diseases they suffer from are due to lead. Among 3,641 printers applying for sick relief, Silberstein[13] found 65 suffering from lead poisoning (1·7 per cent.). Where, however, diagnosis of lead poisoning is based upon examination of the blood, as in Leipzig, the amount of compensation paid by the Sickness Insurance Society has diminished considerably. Thus, of 207 compositors who were either sent by medical men as cases of, or went themselves suspecting that they were suffering from, lead poisoning, only 17 (8·2 per cent.) showed basophilia to such degree as to warrant the diagnosis. The proportion, on the other hand, among letter founders and electrotypers was 28·6 per cent.
Printers suffer extensively from phthisis, their comparative mortality from this cause as compared with the figure for all occupied males being 290 : 175[14]. This high mortality is probably due mainly to the vitiation of the atmosphere, and reluctance, on account of extreme sensitiveness to draughts, to admit fresh air. This closing of windows by persons employed should be an additional reason for checking the vitiation from Bunsen burners in connection with linotype and monotype machines by the only practicable means of preventing perceptible draught—namely, exhaust ventilation.
[15]—The steel file to be cut is placed on a stone block in the centre of which is inserted a smaller steel block, called a “stiddy.” The worker holds in his right hand a hammer,[257] weighing sometimes 7 or 8 pounds, and in his left, closely gripped, a chisel. Each tooth in the file—and there may be as many as 3,800 teeth to be cut—is the result of a blow on the chisel, and we have counted as many as 120 blows with a 7-pound and 200 with a 4-pound hammer per minute. To offer resistance to the blow and yet prevent a recoil, the file (in the case of the finer kinds) is placed on a lead bed—that is, a thin strip of metallic lead. With attrition from repeated impact the lead bed becomes worn away in the course of a few days, and part of what is so worn away necessarily takes the form of lead in fine particulate state.
—Absorption of lead follows from the dust generated by each blow, from brushing the dust off the cut file, and from licking the finger and thumb holding the chisel. Other conditions predisposing to plumbism before the present regulations came into force, and not altogether without effect still, were—too close proximity of one stock to another, defective ventilation of the (frequently) small shed in which the work was done, overcrowding, accumulation of dust on the benches, uneven floors, inadequate washing facilities, and, apparently, lack of appreciation of the danger.
The remarkable feature of plumbism in this industry is the long duration of employment before pronounced symptoms manifest themselves (see p. 51). The insidious onset is, however, accompanied by an undermining of the constitution, showing itself eventually in atrophy of the muscles, especially of the thenar and hypothenar eminences of the hand, and of the lumbricals and interosseous muscles of the fingers, the result of continual gripping of the hammer and chisel, chronic interstitial nephritis, with its associated arterio-sclerotic changes, and heavy incidence of phthisis.
Provision of locally applied exhaust ventilation has never been suggested for this industry, owing to absence of power to drive a fan in the small workshops, and because no lead dust is seen to be given off. Diminution in the number of cases is due to the fact that machine file-cutting (with zinc as the bed) has been substituted for hand file-cutting for coarse files. In hand cutting, in some instances, beds of pewter, or of alloys with comparatively small proportion of lead, have replaced the use of lead beds. The remedial measures prescribed in the regulations have also played a part.
At the time the regulations came into force, in 1903, there[258] were about 708 file-cutting shops in the United Kingdom, of which 517 were in Sheffield. Immediately after they came into force 126 certificates of exemption were granted for use of beds containing less than 5 per cent. of lead, and every year fresh applications are received[16]. There does appear, however, to be difficulty in securing beds which conform to the standards laid down. Thus, in the four years 1907-1910, of 23 samples submitted as containing less than 5 per cent., 16 were in excess.
The number of reported cases of poisoning in the five years 1900-1904 was 151, and 51 in the five later years 1905-1909. The attack rate is about 10 per 1,000, but, although this may be low compared with other trades, the much greater severity of the attacks has to be borne in mind.
—The process consists in keeping the files in a bath of molten lead at high temperature, covered with charcoal. The file is removed when red-hot, straightened if necessary, and plunged into a solution of brine.
—Poisoning is attributable to fume given off by the molten lead (a temperature of 850° C. was recorded by S. R. Bennett—see p. 201), risk of which can only be met by efficient hooding and exhaust unless an alternative method of hardening is adopted, dispensing with the lead bath—e.g., by exposure to heat on a hearth, in a gas furnace, or by other means. In this small industry in and around Sheffield three cases were reported within a year, each involving partial or complete paralysis of the extensors of the forearm. Of ten men employed at the work, three showed presence of a blue line, three were cachectic, and one had weakness of the arms and wrists. In two of the three factories attempts had been made unsuccessfully to carry the fumes away by a hood and duct[17].
Hardening of forks and similar articles in the same manner has also given rise to poisoning.
We are informed that methods of hardening and tempering drills, tools, etc., by the use of fused metallic salts, are being adopted in some works. By mixing together two or more salts in definite proportions, suitable fusing-points can in all cases be produced. These baths can be raised to any desired melting-point to suit the requirement of different steels. For example, sodium nitrate and potassium nitrate in certain proportions give melting-points from 220° to 340° C., and can be used for tempering baths up to 600° C. For tempering above 600° C.[259] mixtures of sodium chloride and potassium chloride can be used, whilst for hardening, sodium chloride or barium chloride give adequate ranges of temperature. Similarly, mixtures of sodium sulphate (melting-point, 890° C.) and lithium sulphate (860° C.) can be made to give any melting-point from 605° to 860° C.
[18]—Cheap hollow-ware vessels such as kettles and frying-pans are often coated with a mixture of (usually) half lead and half tin by dipping them into a bath of the molten metal, after cleaning in hydrochloric acid.
—Duckering has shown (see p. 203) that fumes of chloride of lead are given off which are especially noticeable when the dipped article is removed to a stand to have the superfluous metal wiped from it with tow while still in a molten state. Detailed reference is made on p. 204 to the nature of the fume given off and the amount of lead present in the atmosphere breathed and inhaled daily by the worker.
The danger from fumes can be in great measure removed, both from the bath and from the wiping stand, by locally applied exhaust ventilation, which may be secured by utilization of the draught from the fire under each melting-pot, or by a hood and duct carried vertically through the roof if arranged as described on p. 209. Danger from dust arises also from the skimmings, if not deposited in a receptacle within the hood, from dust and débris on the floor, and possibly from traces of metallic lead and lead chloride attached to particles of tow floating in the air. Risk of lead absorption is less in later processes, such as affixing the spout and handle (mounting), and hammering or denting. Occasionally roughnesses are removed by rubbing the coating with emery paper.
—Hames, buckles, bits, etc., are usually coated with nickel or copper, more rarely with silver. The process is in the nature of soldering, and the steel, prepared in the same way as has been described for hollow-ware, has a mixture of two parts tin and one part lead poured over it on a hearth. The strip of thin nickel sheeting is passed through a similar mixture, and is wiped with tow—the operation to which such poisoning as occurs in this industry is mainly due, in consequence of the difficulty of efficiently removing the vapour of lead chloride from the molten metal upon the long strip of nickel. Subsequently the prepared steel article and the strip of nickel or copper are made to unite under pressure of a soldering-iron.[260] In silver plating danger from fume is slightly less, as the steel portion (for example, the hame) only is tinned. In the final operation of polishing on a mop danger arises from dust, unless locally applied exhaust is provided.
—Use of a tinning mixture of lead and tin in this industry is obviously in the nature of soldering. The body of the drum is made either of black sheet-iron or of terne (lead-coated) sheet. In order to unite the seam and to fix the bottom sheet, the drum is made to stand in a shallow bath and laid on its side. The danger from lead chloride vapour is considerable, and the method of prevention is precisely of the kind described above.
Similar coating of articles first cleaned in hydrochloric acid is met with, as, for example, of the component parts of radiators of motor-cars, of steel bars, and of wire. Incidence of poisoning has not occurred to an extent to make necessary more than the application of locally-applied exhaust to remove the fumes.
—The manufacture of lead-coated sheets for roofing purposes is carried on in a few works in South Wales along with the manufacture of tin plates. Lead poisoning in the industry is practically unknown. We can only recollect occurrence of one case, despite the fact that the mixture contains from 65 to 95 per cent. of lead. For cleaning the plates prior to passing them through the concentrated zinc chloride flux into the molten mixture, dilute sulphuric acid is used, and not hydrochloric acid. As to the remarkably different results on health of the two processes, Duckering concludes:
“The absence of lead poisoning among terne-plate workers and tinners would appear to be explained by—(1) The use of cleaning agents and a flux of such a nature, and in such a way, as to involve a minimum contact with the tinning metals, and under such conditions as to inhibit extensive interaction between them, and also under such conditions as to inhibit production of fume or vapours, even if any interaction occurs; (2) use of a scientifically prepared flux containing no uncombined acid or excess of water in such a way as to prevent introduction of these substances, or of ferrous compounds coming into intimate contact with the tinning metal; (3) so conducting the operation of tinning that any chlorides possibly adhering to the plates are removed before the plate reappears in the open air, under conditions preventing them from appearing in the air as[261] vapour, but it is very doubtful whether any chlorides could adhere to the plates; and (4) absence of any manual work on the plates before the metallic coating is set and hard. On the other hand, the existence of widespread lead poisoning in tinning of hollow-ware is explained by—(1) Use of cleaning agents and flux in such a way as to bring these materials into intimate contact with the tinning metals under conditions eminently favourable to chemical interaction and vaporization of resultant compounds; (2) use of an unscientifically prepared flux containing a large excess of water and much free acid; (3) so conducting the operations as to favour the escape into the atmosphere of vapours of soluble lead compounds, such as lead chloride, and of metallic lead and soluble lead compounds, carried mechanically by fibres of tow during processes subsequent to tinning, as in wiping; and (4)—a minor point not to be lost sight of—possibility of contamination of the hands by soluble lead compounds, due to manipulating the material with which the articles are wiped.
“It should be added that while use of a scientifically prepared flux [(2) above] in hollow-ware tinning would no doubt lessen the possibility of the production of fumes, it is not to be anticipated (unless a flux containing no chlorides were used) that this would do away with lead poisoning. In other words, the method of use is, as indicated above, a far more important factor.”
In the ten years 1900-1909 the number of reported cases in tinning hollow-ware was 93 among about 200 persons employed, in harness furniture 23 among about 150 persons employed, and in iron drums and kegs 47 among about 250 persons employed.
—The figures included in the table on p. 47 have reference only to these processes as carried on in factory premises. House plumbers, when reported, are included with house painters. The figures are made up of two classes—(1) Those handling white and red lead paste, and (2) those engaged in soldering and lead burning. The number of cases reported in the ten years 1900-1909 was, in the first class 122, and in the second 95.
Any worker using red lead as a jointing paste who is not a house plumber or a coach or ship builder is included under the first heading, as, for example, electricians, persons engaged in mechanics’ workshops, lead-light making where red lead cement is brushed between the lead lines and the glass to render them[262] water-tight, and such occupations as placing strips of canvas coated with red lead between sheets of iron work before riveting, so as to afford protection against rust. In several reports there is reference to the dust created in the breaking up of old joints with aid of hammer and chisel before proceeding to recaulk them.
Dust in making up the paste is the principal source of danger. This is crudely done, and unless large quantities of paste are made exhaust ventilation is never provided, in view of the intermittency of the work. The wearing of a respirator should be possible, but it would be unsafe to recommend that as a sufficient means of prevention. Installation, whenever possible, of localized exhaust ventilation at the mixing bench is most desirable. Personal cleanliness is important, as the hands become ingrained with the paste.
The heading “Soldering” includes in the main (a) the soldering of tins of all descriptions, bicycle lamps, etc., with a stick of solder, either held in the hand or lying on the bench, which is touched by the hot soldering-iron, the surface to be soldered having previously been cleaned with “killed spirit”—i.e., zinc chloride flux; and (b) lead burning, by means of a hydrogen or oxy-hydrogen blowpipe flame, of lead-lined boxes, vats in sulphuric acid and other chemical works. Some cases are included which occurred in the manufacture of solder itself. Green wood is held at the bottom of the pot of molten metal, and the gases distilled from the wood pass upwards through the metal and escape at the surface, carrying small quantities of lead into the atmosphere of the workroom.
—It can be confidently said of soldering that, bearing in mind the very large number of persons employed, the number of cases reported is remarkably small, and it is difficult to assert generally, as can easily be asserted of tinning, that inhalation of soldering fumes must necessarily set up lead poisoning. Moreover, examination of persons employed in soldering for signs of lead absorption is almost always negative, a blue line on the gums even being rarely visible.
On the other hand, the process of soldering is so analogous to that of tinning that such poisoning as occurs is probably due to inhalation by susceptible persons of lead chloride fumes. And this is borne out by the results of analysis in the Government laboratory of a sample of deposit collected from a duct where exhaust ventilation had been applied to take away the fumes.
[263]
The material was a black mass, obviously containing a large proportion of carbon.
On completely extracting with water, the solution was found to show an acidity equal to 0·20 per cent. of hydrochloric acid calculated on the original sample, and in this solution the following metallic substances were present—viz.:
| Percentage on Original Substance. |
|
|---|---|
| Zinc, calculated as zinc chloride | 19·53 |
| Copper, calculated as copper chloride | 1·77 |
| Lead, calculated as lead chloride | 0·19 |
Tin and arsenic were both absent, and the chlorine present closely corresponded with the proportion of chloride shown above.
The portion of the substance which was insoluble in water was found to contain the following metallic substances:
| Percentage on Original Substance. |
|
|---|---|
| Tin, calculated as tin oxide | 6·09 |
| Lead, calculated as lead oxide | 1·33 |
| Copper, calculated as copper oxide | 0·57 |
| Zinc, calculated as zinc oxide | 0·20 |
This portion of the sample was also free from arsenic.
We believe that where soldering is done by several persons in a workroom, inhalation of the fumes is prejudicial to health, and that the usual methods of localized ventilation are desirable. Where this has been done the result has been in every way satisfactory.
In lead burning the heat from the blowpipe flame is sufficient, if kept long enough in contact with the lead sheet, to cause volatilization of the metal, and, as the worker’s face must necessarily be close to the flame, inhalation of fume is inevitable. Such work, however, has often, unfortunately, to be carried on in confined spaces where exhaust ventilation cannot be applied.
[19]—The malady the brass caster has suffered from in the past is par excellence brassfounders’ ague. Lead, however, is introduced (rarely exceeding 10 per cent.) for the purpose of softening the alloy of copper and zinc. Of 77 cases of lead poisoning in the ten years 1900-1909 included under the heading “Brass,” 38 were polishers, 28 casters and others, and 11 chandelier fitters. Cases occur among the casters probably from inhalation of the fumes in pouring, and among the polishers from inhalation of the small proportion of lead in the dust given off in the absence of adequate exhaust. In a factory where there were two emery wheels, one with a hood and fan to carry the dust away, while the other remained unprotected, the worker at the unguarded wheel suffered from lead poisoning. In filing and dressing the article is held in a clamp with leaden claws,[264] which gradually become worn away, just as does the lead bed used by the file-cutter. This may account for the poisoning reported among filers and dressers.
A sample of dust taken from under a calico mop for brass polishing was found in the Government laboratory to contain 2·1 per cent. of lead.
The joints of chandelier fittings are sealed with a white lead paste. Instead of always testing the completeness of the seal by means of an air pump and pressure gauge, the fitter frequently tests it by applying his lips to the unsealed end and blowing through the pipe. All the cases among chandelier fitters are caused in this way—perhaps the clearest instance of poisoning by absorption through the alimentary canal, as distinguished from absorption through the lungs, that can be cited. While use of an air pump and immersion of the joint in water or pressure gauge only is an entire protection, and should be provided wherever this work is done, constant supervision as to its use is called for. The sealing of the joint can be done with a material known as “caulkite,” containing neither white nor red lead.
For references, see end of Chapter XVII.
[265]
[20]—The usual method in this country is that known as the “Dutch process,” although the German chamber process, precipitation processes, and others, are all practised.
—A layer of spent tan is placed on the floor of the stack (a chamber with walls of brick some 25 feet high, and a vertical opening from top to base through which the men enter), upon which are arranged earthenware pots partially filled with dilute acetic acid. Strips of lead are then placed on small square “cockney” pots, or more rarely in the form of folded grids, inside deep, long “castle” pots, and the whole covered with boards, resting on special “bearer pots” containing dilute acetic acid. Ten to fifteen of such layers (blue beds) one on the top of the other are built into the stacks to a height of some 20 feet. When completed, the stacks remain for 80 to 100 days before being emptied. During this period the temperature rises to 75° to 80° C., considerable evolution of carbonic acid gas takes place, and the lead is converted first into acetate and subsequently the white basic carbonate. The layers (white beds) are uncovered and the corroded strips (corrosions) collected by hand. They are placed in trays and carried to heavy steel rollers by means of which and subsequent raking in wash becks the carbonate is detached from the uncorroded central core of blue lead. In many factories corrosions are now conveyed from the stacks to the wash becks or rollers by travelling cranes. The recovered blue lead is removed in a wet state to be remelted and recast. The corrosions, after passing through the rollers and wash becks, are shovelled on to a picking-board and transferred gradually to the grindstones. From the stones the ground pulp[266] passes to the settling becks through several gratings of fine copper mesh. In the form of pulp the material is ladled by hand into bowls for conveyance to the drying stoves. When dry, the contents of the bowl are emptied into barrels and headed, or into hoppers, from whence the material is conveyed to be packed either by hand or automatically by mechanical packers, or to be converted into paint.
—In casting the strips, risk does not arise from lead fume, as the temperature at which this is effected (350° C.) is too low for appreciable fume to be given off. Danger here is from skimmings and deposit of them on the floor or into a receptacle unprovided with exhaust draught. Pots in which remelting of the uncorroded cores (returns) is done should be provided with hoods and exhaust, because of the dust given off in stirring and skimming and the spurting which occurs as they are thrown in wet. In making the blue beds, dust arises from particles of white lead adhering to the pots and in the tan bark. Pots, on removal from the white beds, should have all white lead inside them removed by washing in a tank. Screening of the bark should be dispensed with. Emptying the white beds accounts, perhaps, for the largest number of cases, owing to the impossibility, in the present state of knowledge, of dealing with the dust by means of exhaust ventilation, or quite adequately by watering or wearing of respirators. Watering by means of a hosepipe with rose attached is, however, the main safeguard. Substitution of the square cockney pots for the long castle pot is also of moment, as the flat plates of lead form a denser and more porcellanous corrosion than that of the grids in the castle pots. Moreover, in stripping the beds the flat corrosions can be lifted into trays without creating dust, whereas to dislodge the contents of the castle pots may require a sharp tap, and the unglazed portion of the interior surface of the latter retains some carbonate when moistened. Watering requires to be thorough and done with care, or else the softer material of the corrosion may be washed into the tan. No less important is it to water the layers of tan, and at a time while they are yet warm and slightly damp, otherwise the tan becomes so dry that the water runs through, and does not adequately prevent dust formation on its removal. A requirement of the special rules is that the trays for collecting the corrosions shall not stand directly upon the beds. When corrosions contain an undue proportion of lead acetate, they are termed technically “floury,” and much dust[267] may arise from them on watering unless this be done with a very fine rose.
Dust at the rollers and wash becks is usually checked by preliminary immersion of the tray of corrosions in a trough of water, but the extra weight of the water causes this sometimes, in the absence of mechanical arrangements for immersion, to be done perfunctorily. Where there are rollers, the tray is inserted in a small opening above them, the contents saturated by a spray, and then tipped over. This method also may fail to control the dust, as, unless the men engaged in washing the corrosions in the wash beck keep the mass tipped in from piling up in a heap, the contents of the trays are discharged on to the heap, and not into water. In some factories exhaust ventilation at the rollers or wash becks has been necessary.
During subsequent wet processes of grinding danger is mainly from splashing. From the settling tanks the white lead is pumped into filter presses, and the resulting cake is dried. Here the considerable risk from splashing is again almost unavoidable. Concrete floors are necessary. Emptying the stoves involves much handling, with inevitable creation of dust, especially when the bowls are withdrawn from the racks. Risk has been greatly lessened by reducing the height of the shelves to 10 feet and prohibiting the piling of one bowl upon another. Mechanical drying stoves into which men need not enter either for filling or drawing are now commonly met with. Of these there are various types—(1) Horses similar to those common in laundries, which can be withdrawn on rails; (2) small chambers built up one upon another somewhat in the form of gas retorts in a gasworks, heated by steam jackets and coils, each chamber containing only two or three cakes of white lead pulp, the cakes themselves being removed by a mechanical process from the press into the drying chambers; (3) bogies carrying the white lead in bowls on racks made to pass through the tunnel-like stove; (4) drying machines—i.e., closed cylinders fitted with a series of platforms so arranged that they may be charged with white lead on one side, and so fixed as to be turned round by means of mechanical appliances. When dry, the material is discharged into a chute by a series of scrapers into a small enclosed compartment, holding the barrel to be filled. With the drying machine, however, there is considerable risk of dust leakage, especially when the doors are opened. In packing by hand, safety depends on efficient exhaust ventilation when the[268] contents are tipped into the barrel, but a danger constantly present is that, to get through the work quickly, the bowls may be withdrawn from the influence of the exhaust before the last traces of dust have been removed from the bowl. Mechanical packing, by means of a large bladed screw forcing the white lead into the barrel, which as it becomes filled is lowered automatically, is everywhere desirable. An essential condition of this method is that a dust-proof collar should connect the automatic packer with the barrel. Some dust almost inevitably escapes, and a hood and exhaust should be provided, however perfectly the machine is said to act.
Much of the white lead is converted into paint on the premises, being ground in oil either in pug mills, Torrance mills, or under edge-runners. A negative pressure must be maintained inside the casing, which must enclose the stones. Here the conditions are precisely those described under the manufacture of paints and colours. In some white lead works conversion into paint is done without the dangerous process of stove drying, either by drying under a vacuum or by mixing the white lead directly with oil. In the process of grinding the oil incorporates itself with the white lead, and the water is forced out, running away in a clear stream.
—In this method, almost universally used in Germany, and adopted in at least one large white lead works in this country, a chamber arranged with numerous sets of parallel bars on which the thin strips of lead are set saddle-wise takes the place of the stack in the Dutch process. Carbonic acid gas and acetic acid vapour act on and corrode the strips. In a period of from eight to ten weeks the corrosions mostly fall to the ground. Such of the strips as do not fall have to be lifted off the bars, having been previously well saturated with water from a hosepipe, and are dropped on to the floor of the chamber. We are not satisfied that working in the dark, confined chamber by artificial light is less dangerous than working on the stacks. Chamber-made lead undergoes practically the same subsequent processes as have been described.
—These also dispense with stacks and the consequent risks attending work on the blue and white beds, but they substitute another—namely, use of oxide of lead (litharge or the suboxide) as the initial product to be carbonated, with the inevitable danger, in the absence of mechanical contrivances entirely closed in, of shovelling dusty material. In[269] many of these methods, however, mechanical arrangements obviate hand labour or contact with dust in all but the first process.
The Brimsdown process[21], for instance, is automatic and free from dust, except in the initial stage of preparation of litharge in cupellation furnaces. The great risk from the disintegration of this material (see p. 250) by turning it out on the floors is obviated by allowing disintegration to take place in the pots (which must not, therefore, be completely filled), and tipping these when cooled directly into a breaker under powerful exhaust draught. From the bin into which it falls the material is conveyed by dust-proof elevators to (a) screens and packing arrangements when the object is flaked litharge, or (b), in the case of the bulk of the material for manufacture of white lead, by enclosed conveyors to reducers and mixing mills, where reduction and hydration take place. It is then charged automatically into weak solution of acetic acid, and by agitation with carbonic acid gas converted into basic carbonate of lead. From the carbonators pumps force it into filter presses, where the acetate is drained off and washed out by pure water. The cakes of white lead are fed into mixing machines and pugged with linseed-oil until the water has been entirely removed, and finally passed through the roller mills to be packed in casks.
Dry white lead is made by feeding a metal travelling lattice with pulp white lead inside a drying chamber entirely closed in. When dry, the white lead is automatically brushed off, elevated, and automatically packed in a chamber under efficient exhaust draught. In this part of the process, therefore, risk to the workers is very small.
The stringent Special Rules for the White Lead Industry show what other precautions, in addition to exhaust ventilation, are necessary—especially personal cleanliness. The effect of one other factor—casual labour—however, must be referred to. The condition at the present time is very different from that which existed twelve years ago. From one factory in 1899, depending much on casual labour, 111 cases were reported, and from another 72. In an inquiry made by one of us in 1898, information was obtained of the actual number employed on any one date, and of the total number passing through the factories in a year.
Among the firms with regular employment at that time, the incidence of lead poisoning was 60 per 1,000 on the average number employed, and in those with casual employment 390[270] per 1,000. Work in lead had secured a bad name, and no one who could get employment elsewhere would take to it. Consequently, the class of men applying for work was a low one—men discharged from other employment and those unfitted for skilled labour. Not a few were addicted to alcohol. The work was unskilled, and had the additional advantage to men of that class that much of it was piece work, paid at a good rate, which could be finished as a rule by three o’clock in the afternoon.
Diminution in the number of cases from 399 in 1899 to 34 in 1910 has been brought about mainly by—(1) Improved structural conditions; (2) adoption of mechanical means (cranes, rails, hoists, etc.) for conveyance of material in substitution for hand carrying; (3) exhaust ventilation, where dust arises as in packing and paint-mixing; (4) periodical medical examination; (5) diminution in height of the stoves or adoption of mechanical drying stoves; (6) conversion of white lead into paint by means of direct mixture with oil while in the pulp stage; and (7) substitution of small, square, glazed pots—cockney pots—requiring the lead strips to be placed on them, for the deep castle pots into which the lead grids are folded in the white beds. Prohibition of female employment in the dangerous processes was made prior to the Special Rules of 1899. Their greater susceptibility as compared with men, the special effect of lead on the uterine functions, and the unsuitability of much of the work for women, fully justified the step recommended by the White Lead Committee in 1898.
[22]—The industry includes the manufacture of earthenware, china, tiles, majolica ware, Rockingham ware (teapots), sanitary ware, china furniture, and electrical fittings, and any other articles made from clay; but of the total 6,865 persons employed in lead processes in the whole of the United Kingdom in 1907, 5,834 are included in the manufacture of the first three. And even in the manufacture of earthenware, china, and tiles, the poisoning which occurs is not distributed evenly over the whole of the factories. These number 550, and, taking the period 1904-1908, 5 potteries were responsible for 75 cases, 17 for 119, and 151 for 323, leaving 377 factories out of the 550 from which no case was reported. Incidence seems to depend more on the scale and rapidity of the output of cups, saucers, plates, and tiles, in everyday use, than on anything else.
The number of reported cases year by year from 1900 to 1909 has been as follows:
[271]
ALL LEAD-WORKERS IN PLACES UNDER EARTHENWARE AND CHINA SPECIAL RULES. WHOLE OF UNITED KINGDOM (INCLUDING NORTH STAFFORD).
Number of Persons Employed.
Number of Persons Employed.
[272]
The processes in so far as lead enters can best be divided into—(1) Glaze; (2) decorative.
1. Glaze Processes.—The charge of glaze is made by weighing out and mixing carbonate of lead with the necessary silicates and silico-borates in the lead house or mixing-room, where wet grinding prepares the mixture for the dipping-tub. “Putters-up” hand the ware to the dipper, from whom “takers-off” place it on boards for removal to the drying still, or place it (in large works) directly on to the shelf of an appliance known as a “mangle,” in which an endless chain carries the ware through a heated chamber. Subsequently superfluous glaze has to be removed from the base, rims, and not infrequently also other parts of the articles. This ware cleaning is performed with a wet sponge or flannel, either while the ware is still moist or by scraping, the particles removed dropping into a vessel of water; or, if the glaze is dry, over a grating provided with exhaust draught. The ware is next removed by the glost-placer on boards, and each piece is separately placed by him in the sagger (fireclay receptacle) and carried into an oven to be fired.
2. Decorative Processes.—Majolica painting is the application of a coloured glaze rich in lead by means of a brush. Ground-laying consists in dusting powdered enamel colour on to a pattern first printed on glazed ware with an oily medium. Colour dusting differs from the same only in detail.
Aerographing (colour blowing) is the blowing on to the ware, by means of a jet of compressed air, coloured glaze, or enamel colour held in suspension in oil or other liquid in a glaze kettle or aerograph instrument.
—Apart from risk inseparable from, and increased by, defective lighting, uneven floors of wood or brick, collection of dust on benches and floors, and the risk entailed in the sweeping of these even when watering is practised, and lack of care and attention to detail on the part of the worker, the following special dangers are incidental to the various processes: In dipping the glaze (except in tiles, where the surface only is allowed to touch the liquid), splashes on to the face and overalls of the dipper, “hander-up,” and “taker-off” (dipper’s assistants), and “threader-up” (in the case of china furniture), especially when, as with plates, there is much shaking of the ware. These splashes dry, and the overalls may become so coated with glaze that every movement, such as carrying boards[273] or leaning against the mangle, crumbles it off as dust into the air. As the dipper shakes the ware, some of the drops are disseminated into the atmosphere as a fine spray. In ware cleaning the work may have to be done so rapidly that it is difficult always to observe proper care, and the worker is tempted to withdraw the article from the range of the exhaust. Sometimes a ware cleaner is seen blowing away with her mouth dust lying on the ware.
Dipping-boards, unless freed from adherent glaze by washing after use, create dust whenever ware is placed on, or removed from, them, when they are handled and placed on or taken off the stillage bars, and when they are stacked. Persons gathering at the mangle are exposed to dust if there is any outward current of air from it. The glost-placer raises a slight amount of dust as he takes the ware from the board and places it in the sagger. The dangerous practice formerly almost universal of rubbing the bottoms and rims of cups, etc., either together (without use of an exhaust) or rubbing them on a piece of leather fixed round the chest, is generally replaced by removal of the glaze on a moist piece of flannel, but it is still possible to find men doing it in outlying potteries. In majolica dipping and painting (apart from the obvious risk of splashing and contamination of the hands), danger arises mostly from scraping the edges and under surfaces of the tiles on to which glaze, when applying the background, has overflowed. The amount of glaze so removed is considerable, and if it is not all caught in the trough of water, the floor becomes an added source of danger.
In all the decorative processes—ground-laying, aerographing, colour-dusting, and grinding of colours for aerographing, etc., the danger is one solely arising from dust.
—Meticulous attention to detail, not only in the provision, but also in the maintenance, of the locally-applied exhaust ventilation, alone can allay the danger in the processes to which dust is incidental, such as ware cleaning, gathering at the mangle, glost-placing, and the decorative processes. The Lead Committee considered that, as there was no rapid method of testing the actual degree of moisture, exhaust ventilation might be required in the case of ware that was not cleaned within fifteen minutes of the application of the glaze. Such a requirement would prevent the practice now prevalent of painting as many as three dozen tiles, piling them one on top of another,[274] and then proceeding to the operation of scraping. No danger attaches to removal of glaze with a damp sponge or flannel, but means must always be at hand for washing and damping them. In the dipping-house, (a) impervious floors should be provided, which could be washed down so as to prevent the risks from sweeping, and from glaze drying, and being raised as dust; (b) partial covering of the dipping-tub to prevent splashing and spray; and (c) substitution for the overalls at present worn by persons in the dipping-house, glost-placers, millers and mixers of glaze, majolica paintresses, and others, of overalls of some light waterproof material which could be sponged, or of aprons of waterproof material worn in front of the overalls. Dipping-tubs and walls and floors in close proximity to them can with advantage be painted red. Dipping-boards should be washed with clean water after every time of use. Automatic machines for washing and scrubbing boards are in use in some factories.
To reduce risk or remove the danger of lead poisoning in this industry, use of low solubility glazes or of leadless glazes are advocated. On this point the Lead Committee say: “The effect of melting the lead with silicious matter amounts to imprisoning it in such a manner as to render it less liable to the action of the acids which it meets in passing through the human body, and in consequence largely reduces the likelihood of its absorption into the blood. If the frit is properly compounded, all but a small fraction of the lead is rendered insoluble, and glazes so made are spoken of as ‘low solubility glazes.’ The finished glaze generally contains from 12 to 22 per cent., or more, of lead oxide, but after the process of fritting with sufficient silicious material only from 2 to 5 per cent. remains soluble.”[A]
[A] Raw lead comprises red lead, white lead, and litharge. If introduced in this form as a constituent of glaze it is soluble in dilute acids. If, however, the raw lead is fluxed by heating with a part or the whole of the silica, it is converted into “fritted lead.” The solubility of the frit depends upon the relative proportions of material taken. Thorpe[23], as a result of numerous analyses of lead silicates (after determining their solubility as regards lead), both simple and complex, in use in the potteries and on the Continent, found that the quantity of lead dissolved had no necessary relation to the quantity of lead in the silicate. “Primarily and in the main the insolubility of the lead depends not upon any one oxide or group of oxides, but upon the maintenance of a certain proportion between the whole of the basic oxides on the one hand and the whole of the acidic oxides on the other. If the value of ratio bases/acids is higher than, or approximately equal to, two, the amount of the lead extracted is small, but if it fall much below two, the quantity of lead dissolved begins rapidly to increase.”
On the subject of the use of leadless glazes, the Committee[275] conclude that in all classes of pottery ware a great many articles can be manufactured in a very high state of perfection, with reduction in the cost of production of certain classes of common ware, such as jampots and Persian painted ware; but that in certain other classes, owing to the excessive number of “seconds,” their use would entail increased cost or sacrifice of quality, so much so as to involve loss of important markets; and, finally, that certain kinds of ware, in consequence of difficulties relative to accuracy in reproducing old patterns, colours, or methods of decoration, cannot at present be made at all without use of lead.
In the case of manufacturers who are able to conform to the Thorpe test of low solubility—i.e., glaze which yields to a dilute solution of hydrochloric acid not more than 5 per cent. of its dry weight of a soluble lead compound, calculated as lead monoxide (PbO)—important relaxation of certain special rules are allowed, such as limitation placed on the employment of females and young persons, and periodical medical examination of the workers.
H. R. Rogers[24], one of H.M. Inspectors of Factories, Stoke-on-Trent, has worked out a simple test to show approximately how much lead has been used in the glaze of a piece of pottery. Thus, by treating glazes with hydrofluoric acid for forty seconds, absorbing the liquid with filter paper, precipitating the lead on the paper as the sulphate, dissolving out the sulphate soluble in water, and then precipitating the lead on the paper as sulphide, stains are produced varying, in depth of colour, according to the proportion of lead in the glazes concerned (see Plate IV.).
Briefly summarized, the recommendations of the Potteries Committee in regard to the processes are—
Manufacture of Glazes.—No handling of white or red lead without at least 5 per cent. of added moisture, and no weighing out, etc., nor employment in the room, to be allowed within thirty minutes of such weighing out, etc., without the wearing of a respirator.
Lawning—i.e., straining glaze so as to remove insufficiently ground material through a fine lawn sieve—to be done by an adult male only, except where less than a quart of glaze is lawned.
Dipping.—Impervious floors sloped towards a drain to be[276] cleaned by an adult male, after work has ceased, with a jet of water and a mop. Walls adjacent to dipping-tubs to be tiled or painted with washable paint, and cleaned daily. Dipping not to be done where artificial light is necessary during hours of daylight.
Threading-up and Thimble-picking to be done in a room sufficiently separated from any place where scheduled processes are carried on.
Drying Ware after Dipping.—The same requirement as to floors as in dipping-house.
Boards.—To be cleaned with clean water by an adult male after each time that dipped ware has been placed on them and before subsequent use. Boards for use in lead processes to be painted red at the ends.
Mangles.—Ventilation to be so arranged as to maintain a flow of air into the hot chamber from the workroom. Mangle shelves to be thoroughly wet cleansed once a week.
Ware Cleaning.—Local exhaust ventilation to be applied except when the process is carried on entirely with use of wet materials (damp sponges, etc.), or when done within fifteen minutes of application of glaze. Troughs to be provided to collect glaze, and to be cleaned out and supplied with fresh water at least once a week. The floors and standard of lighting to be the same as for the dipping-house.
Glost-placing.—Boards to be treated as already described. Floors to be impervious. Women, young persons, and children to be excluded, except that women to be allowed to place china furniture and electrical fittings.
Majolica Painting and Mottling.—A sponge and clean water to be placed beside each paintress; special washing accommodation in the painting-room or adjoining it; splashes to be removed immediately by wet sponging. Work-benches and floors to be subject to the same conditions as potters’ shops.
Flow Material—i.e., the substance usually containing much lead in the form of powder and placed in the sagger to cause certain colours applied to biscuit ware to run slightly—to be weighed out in front of an exhaust draught and delivered to the glost-placer by an adult male.
PLATE IV

Fig. 1.—No Lead used.

Fig. 2.—Fritted Lead used.
0·9 per cent. solubility.
Fig. 3.—Fritted Lead used.
1·5 per cent. solubility.
13·9 per cent. total lead.
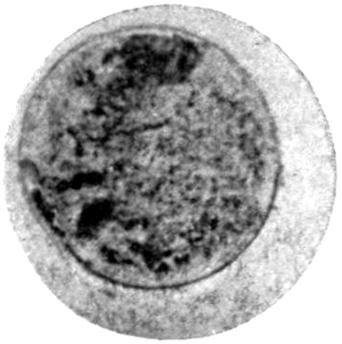
Fig. 4.—Fritted Lead used.
5·0 per cent. solubility.
5·0 per cent. total lead.
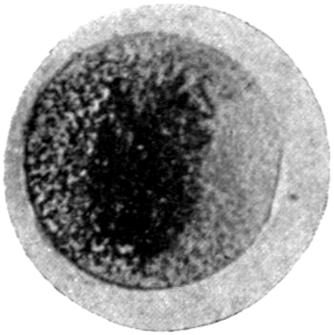
Fig. 5.—Raw Lead used.
19·4 per cent. solubility.
19·4 per cent. total lead.
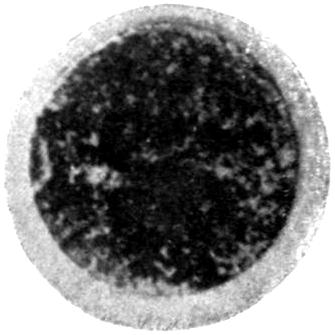
Fig. 6.—Raw Lead used.
44·1 per cent. solubility.
45·2 per cent. total lead.
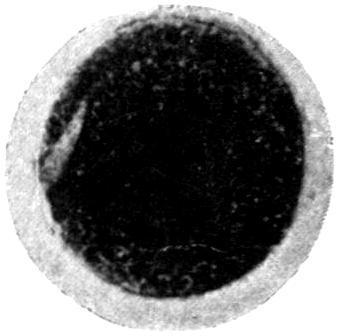
Fig. 7.—Rockingham (Raw Lead) used.
50·9 per cent. solubility.
50·9 per cent. total lead.
Ground-laying, colour-dusting, and aerographing to be done under locally applied exhaust ventilation. Proper receptacles to be provided for cotton-wool used and waste cotton-wool to be[277] burnt. No short-sighted person to be employed to do either glaze or colour blowing, unless wearing suitable glasses, and certificate to this effect to be entered in the Health Register.
[25]—Transfers for the decoration of earthenware and china are made in special factories, of which there are seven, employing 257 persons. The patterns are impressed in the ordinary chromo-lithographic fashion, but as the enamel colours, containing high percentages of lead, are dusted either mechanically in the machine, or by hand by means of a pad of cotton-wool, danger from dust is great in the absence of maintenance of a negative pressure inside the dusting machine and an efficient exhaust draught behind the bench where the final dusting with flour, to remove the superfluous colour, is done. In one factory, before a fresh colour was applied to the adhesive pattern on the sheets, the machines had to be cleaned as far as possible of the previous colour used. To do this it was necessary for the attendant to enter a closed chamber at the back of each machine, so as to supply the powder to the hoppers which feed the rollers, or to clean them by means of a brush, sometimes as often as every half-hour. The upward exhaust ventilation applied to the interior of the machine tended to draw the dust created in brushing past the worker’s face, and led to severe incidence of poisoning. The remedy suggested by Pendock[26] was to dispense altogether with the need for entering the chamber, to maintain a slight negative pressure inside the machine by downward exhaust, and to remove the dust by means of a small vacuum cleaning plant.
At the same factory the flouring bench was in the same room as the machines, and the locally applied exhaust drew its air-supply from the general atmosphere of the room. Apart from faulty arrangement of the exhaust ducts leading to effects of too local a character, dust was drawn from other parts of the room, including the machines, so much so as to necessitate frequent cleaning of the glass hoods. Poisoning among those employed in flouring occurred. To remedy this, an air-grid with curved inlets at intervals of 2 inches apart, leading into a trunk in connection with a fan, was placed along the back of the bench and under the top of the glass hood. In order, however, that its action should not interfere unduly with the general ventilation of the room, but be, in large measure, independent of this, a somewhat similar grid, introducing air from the street outside,[278] was fitted along the front of the bench. The whole arrangement was operated by one suction fan. Ten cases occurred in this factory in the year before this arrangement was carried out. In the three years since, three cases only have been reported. In the ten years 1900-1909, 48 cases were reported among 257 persons employed.
[27]—Surfaces, such as sheet iron for advertisement signs, cast iron for baths and gas stoves, copper for copper letters and tablets, brass for jewellery, and glass for lettering and decoration, are treated with glaze or enamel colours, which, either in the mode of application or subsequent treatment before final vitrefaction, give rise to dust.
In the manufacture of advertisement signs, glaze is swilled on to the sheet of iron. After drying, it is fired or vitrified, and upon this surface as many other coats of glaze are applied as may be wanted. As soon as the colour is dry, lettering is effected by brushing away the dried (but not fired) glaze exposed through stencils.
—Exhaust ventilation for the removal of the dust is essential, but it is, unfortunately, unable to draw the dust away when brushing is done at a distance of more than about 18 inches from the exhaust opening. And some of the plates required are very large. No exhaust-pipe has yet been invented which will follow the hand of the worker without impeding movement. In consequence of severe incidence of poisoning, mainly on young women who do the work of brushing, when the process was first introduced with enamel glazes containing from 15 to 75 per cent. of lead, manufacturers quickly turned their attention to use of enamels free from lead. For this class of work they appear to have been entirely successful, and now lead poisoning is almost a thing of the past. Thus, of 122 samples examined in 1910 from factories claiming exemption from the regulations by reason of the use of enamels containing less than 1 per cent. of lead, excess was found in three only[28].
—The cast-iron bath or stove is heated to redness in a muffle furnace. On withdrawal from the furnace it is placed by the helpers on a table capable of being turned in every direction. Enamel powder is then dusted on to the heated metallic surface through a sieve attached to a long wooden handle, held by the duster, who protects himself from the intense heat by a mask and an asbestos cloth covering.
[279]
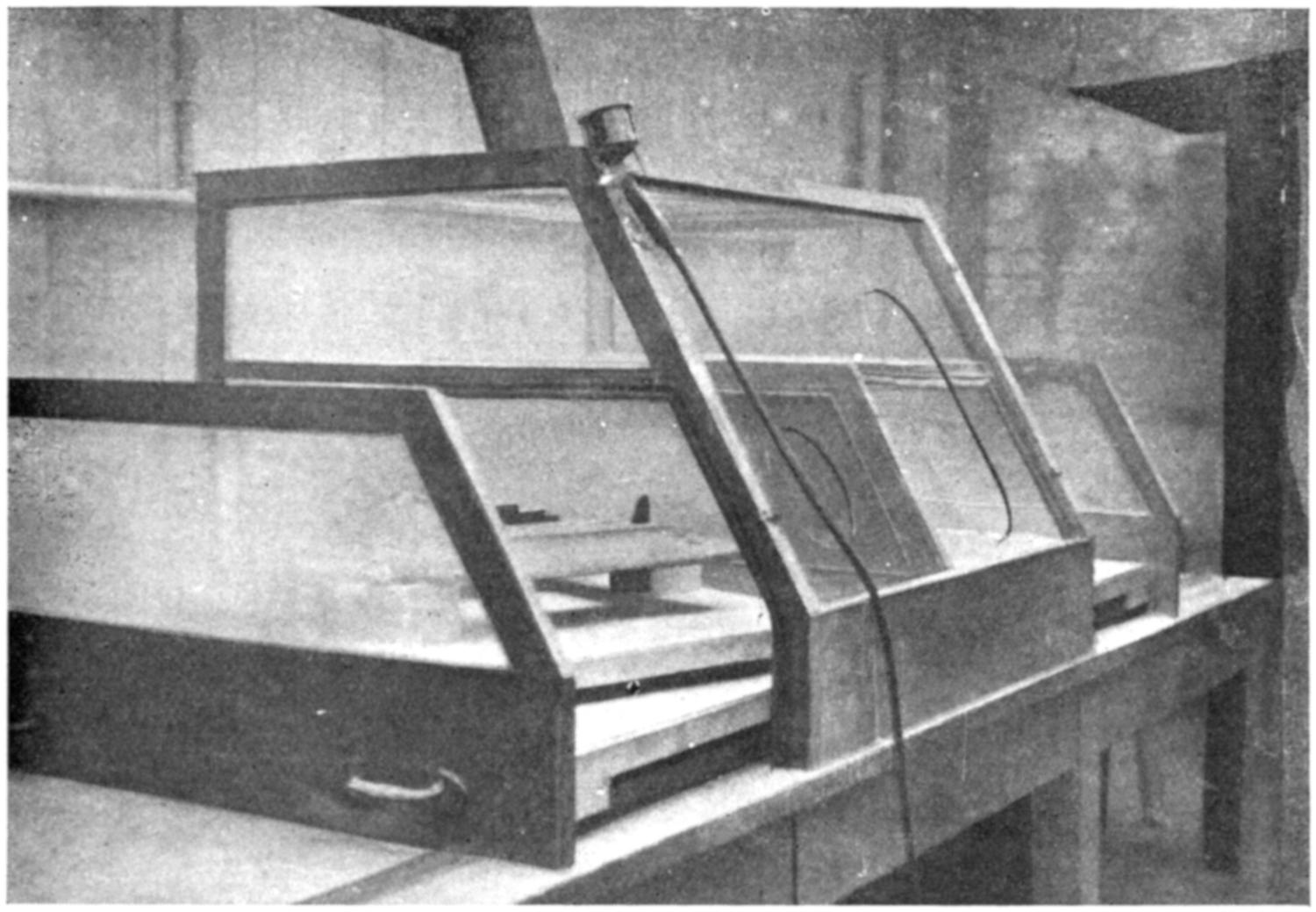
Fig. 12.—The first glaze is sprayed on with an aerograph. The portion of the stove to be glazed is shown on supports on the sliding table, which is half out of the cabinet. When the casting is fully in the cabinet, the end piece and the centre piece close the cabinet sides, and, fitting on a felt beading, make an air-tight joint. The spray, shown in front of the cabinet, is worked through the holes in the glass front. Exhaust is provided at the top.
—The heated column of air carries up much of the powdered glaze as it is unevenly distributed by jolting the handle of the receptacle, and in the absence of very efficient exhaust ventilation this dust will, as the current of air strikes the roof and cools, fall down again. The hood placed over the bath must have steep sides and be brought down as low as is possible without interfering with work, and the duct leading to the fan must be unusually wide, so as to be able to cope with the up-rush of heated air. If the sides of the hood be shallow, not only will the dust fail to be removed, but the hood itself may become so hot as noticeably to increase the discomfort from heat to which the men are exposed during the three or four minutes, five or six times an hour, that the dusting operation lasts. A method has been patented by M. Dormoy of Sougland[29], Aisne, France, for carrying out automatically in a closed chamber the process of dusting on to small red-hot castings, such as are required in the manufacture of stoves. It is not applicable for baths.
[280]
Occasionally, in the case of small castings, again, the enamel is sprayed on by means of an aerograph. For this excessively dangerous process we have seen simple and ingenious devices for carrying it on quite safely in a space under negative pressure, and covered in except for the necessary openings through which to work the spray (see Figs. 12, 13, 14).[A]
[A] The cabinets have been patented by Messrs. Wilsons and Mathiesons, Ltd., Leeds, by whom they are made and supplied. Since using them there has been no trace of illness among the persons employed.
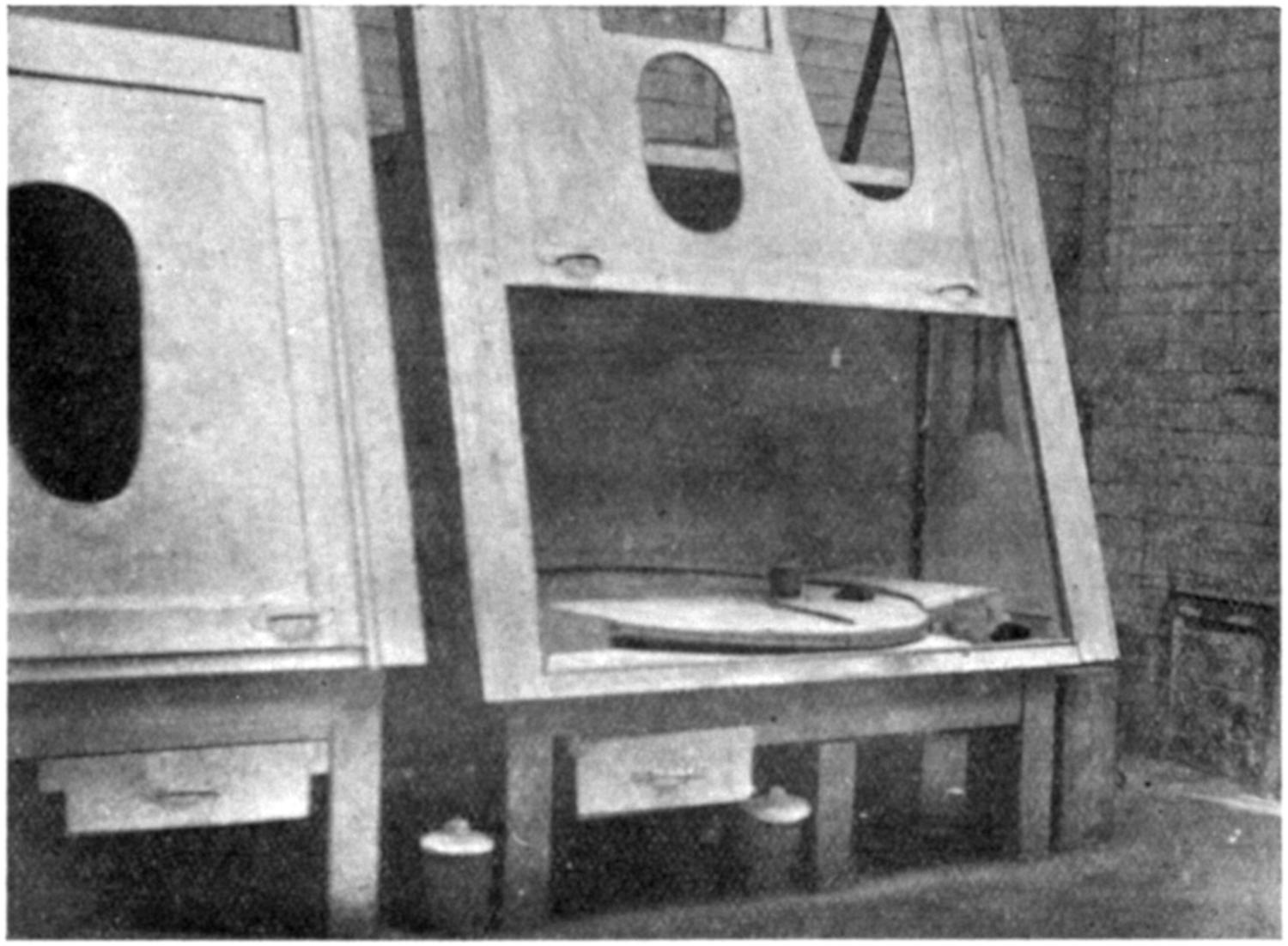
Fig. 13.—After firing the casting is lifted out for treatment with dry glaze, which is sprinkled on with a sifter shown on the table. The turntable enables the operator to manipulate the red-hot casting more easily.
White enamel powders free from lead are used entirely by some firms, but the black and coloured enamels on stove grates contain lead. A frit analyzed in the Government Laboratory was found to contain 26·66 per cent. of lead oxide. The fact that all the lead used is in the form of a silicate, even although the silicate is readily soluble in dilute acid, tends, we believe, to cause incidence of poisoning to be less than might have been expected from the amount of dust often present in the air, and attacks, when they occur, to be less severe, as a rule, than they would be were raw carbonate of lead alone used. For the arduous work entailed the men are specially selected. Despite their exposure to lead dust, the majority continue to work for many[281] years without marked signs of lead absorption. The management should provide a suitable room for the men to cool themselves in the intervals of dusting.
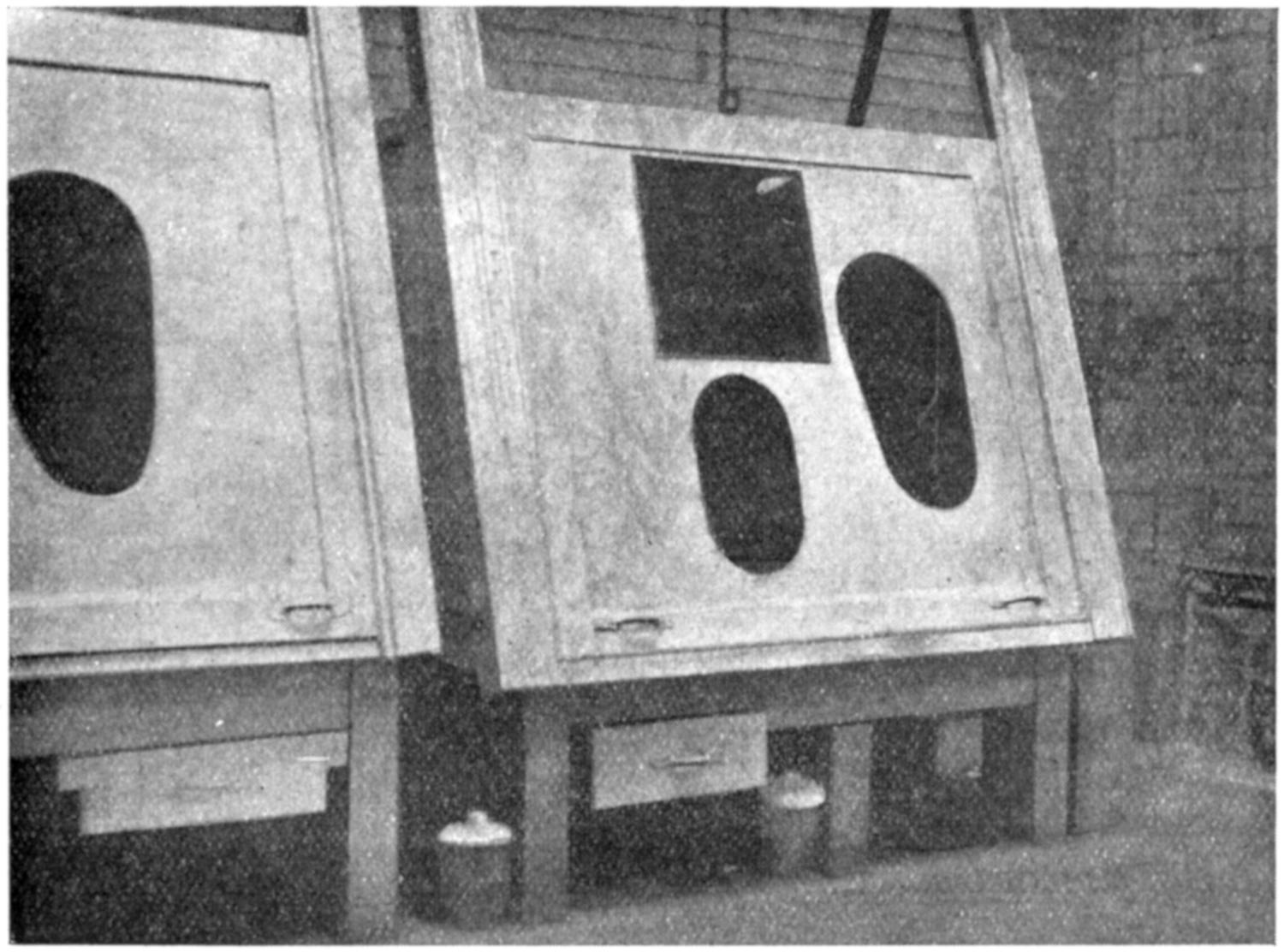
Fig. 14.—The cabinet is shown when dry dusting is being done. The casting is worked by tongs through a slot in the side of the cabinet (not seen), while the worker dusts the casting with his arms through the two front holes. He can see his work through the square pane of glass. (Photographs kindly made by Mr. F. W. Hunt, Leeds.)
[30]—Electric accumulators are secondary batteries which serve for the storage of electricity, in order to allow of a current when desired. A primary battery is one in which the materials become exhausted by chemical action, and, unless a portion or the whole of the materials is renewed, fails to supply electricity. The secondary battery becomes exhausted in the same way, but the chemical contents are of such a nature that it is merely necessary to pass a current of electricity through the battery (charging) in order to recharge them. In the accumulator battery the positive element is peroxide of lead, and the negative element spongy lead. The elements—several positive connected together and several negative—are placed in dilute sulphuric acid contained in vessels of glass.
The form of accumulator in almost universal use now is the[282] pasted plate, but it varies greatly in size, according to the use for which it is required. It may be either large, to act as an equalizer or reservoir of current in electric-lighting installations, or quite small for ignition purposes in motor-cars. The litharge smeared on to one plate becomes converted into the positive element, peroxide of lead, during what is called the “forming process” (passage of the electric current through the dilute sulphuric acid solution in which it is placed), and red lead smeared on to the other becomes spongy lead to form the negative.
The industry gives employment to about 1,200 persons. Plates are first cast in moulds from a bath containing molten lead or of lead with admixture of antimony. Irregularities in the plates so cast are removed by a saw or knife (trimming), and sometimes filed or brushed with a wire brush. The interstices in the plates are next filled in by means of a spatula with paste of litharge or red lead, as the case may be, which has been previously mixed either by hand at the bench or in a special mechanical mixing machine. After drying, the plates are removed to the formation room to be charged. To allow of the passage of the current, positive elements are connected together, and negative also, by means of a soldering iron or, more frequently, of an oxy-hydrogen blowpipe flame. After formation is complete the plates have to be built into batteries, or “assembled.” Tailpieces, technically known as “lugs,” have to be connected with each plate, effected usually by the oxy-hydrogen blowpipe flame. Finally, a connecting bar of lead is cast on or burnt on to the lugs.
—In casting, danger is mainly from dust in depositing the skimmings, and from fume also when old accumulator plates are melted down. For these reasons exhaust ventilation over the melting pots should be provided, embracing also (by branch ducts if necessary) the receptacles into which the lead ashes are thrown. In mixing and pasting, the danger is from dust of oxides of lead to be controlled (see Fig. 6) by—(1) Exhaust ventilation by branch ducts protecting (a) the barrel from which the material is scooped, (b) the mechanical mixer into which the weighed quantity of oxide is discharged, (c) the bench at which the mixing by hand is done; (2) dampness of benches and floor to prevent raising of dust either by manipulation of the (often) heavy plates or trampling into powder the paste which may fall on the ground.
In assembling or putting together of the formed plates, and[283] in earlier stages of the manufacture also, filing or use of a wire brush causes production of metallic lead dust and of the oxides when the brush touches them—a danger only to be met by exhaust ventilation. How far the poisoning to which the lead burners engaged in assembling plates is attributable to lead fume, produced by the high temperature of the blowpipe flame, and how far to handling (with inevitable dislodgment of dust) has not been satisfactorily settled. Incidence of poisoning on this class of worker in the past has been marked.
Generally there is need for impervious floors, solidly built, so as to prevent vibration and the raising of dust from passage of trolleys conveying the heavy plates. Gloves are frequently provided, more to protect the hands from contact with the sulphuric acid used in making the paste and jagged edges of the plates than as a preventive of lead absorption.
In the 10 years 1900-1909 incidence, according to precise occupation, has been—Casting, 33; pasting, 114; lead burning, 69; and assembling the plates, etc., 69.
[31]—Red lead enters largely into the mixture of raw materials for the manufacture of glass. Flint glass, for instance, contains 43 per cent. of lead. The raw materials (white sand, red lead, and generally saltpetre) require to be very carefully mixed, and a few cases of poisoning have been reported from the dust raised in sieving. One man works the sieve, resting on two runners across the bin, while another shovels the mixture into the sieve. The operation is not a continuous one, and respirators have principally been relied on to protect the workers. It should be possible to carry out the mixing operations in a dust-tight closed apparatus.
Poisoning from lead fumes generated in a glass furnace is unknown. Lead poisoning used to be common in the process of polishing cut glass on a brush by means of “putty powder” (oxide of tin, 29 per cent.; and oxide of lead, 71 per cent.), mixed with water to the consistency of a paste. The brush was made to revolve at high speed, with dissemination of the putty powder as a fine spray into the atmosphere of the workroom. Although rouge and oxide of iron have replaced putty powder to some extent—especially for the polishing of the bevelled edges of plate glass—no substitute can at present be found to give the final lustre and brilliancy required in the case of cut glass and in certain kinds of high-class work, such as polishing lenses.
[284]
Locally applied exhaust ventilation has robbed the process of its dangers. Pyramidal-shaped hoods enclose the spindle and putty box and brush before which the workman sits. The draught of the fan prevents escape of spray. The lad who feeds the brush with putty powder stands at the side, and in our experience his cap and clothes are now free from signs of splashing. Formerly the polishing was done by each man at his own berth, thus endangering the health of all working in the vicinity, as the custom of the trade is that the same man carries through the work both of cutting and polishing. Polishing occupies only about a fifth of a man’s time, and it has now, owing to the position of the fan, to be carried out in one particular part of the room.
Dr. D’Arcy Ellis[32], Certifying Surgeon for the Stourbridge district, has described the processes as formerly carried out:
“The mixture of lead and tin is heated over a bright fire in a shallow iron pan. As it melts, the top scum which forms is skimmed off, dried, pounded to a powder in an iron mortar, and afterwards sieved. The person who does this work always suffers more or less. He usually protects himself by wearing a respirator—there is a good draught at the flue, and the sieve is enclosed in a box—but there is always a certain amount of dust. This putty-powder is used on the wooden wheel, and is dabbed on the wheel as it revolves. All good bold work can be polished in this way, and there is not much risk to the workman, as the speed at which the wheel revolves causes the mixture to cling and not fly about. This process does not answer for any fine work, so it is contended; and to enable this kind of work to be properly polished brushes made of bristles are used. They are mounted on an iron spindle, and are usually about 6 inches to 7 inches in diameter, with a face of 1 inch to 1¹⁄₂ inches broad. They are driven at a speed of about 2,000 revolutions a minute. The putty powder is applied to these brushes (which are of various sizes) in the same way as to the wooden wheel—that is, by dabbing it on. For smaller work, such as tumblers and wine-glasses, the workman applies the putty mixture himself, holding the glass against the brush with his right hand, and using his left underneath to apply the mixture. Where, however, larger work has to be done in which the workman cannot manage with one hand, the service of a boy is called in, who does what is called the ‘feeding up.’ This boy stands partly in front and partly at the side of the brush, and applies the mixture with one hand with the wisp of straw. In this position the boy gets splashed with the putty mixture which flies off the brush, and it is generally believed by the workmen to be the most dangerous occupation. At one time—not very long ago—all the various processes of the work were done indiscriminately in the workshop, and consequently the men were frequently found working in a perfect haze of fine dust, which had been thrown off from the brushes. There was no attempt made to separate and detach the less injurious part of the work, such as the roughing and cutting, from the general workshop, the lead polishing only occupying about one-fifth of the workmen’s time. After the glass has been polished by the putty it is taken away to another department, where girls are employed as ‛wipers out.’ They take the glass with the dried putty upon it, dip it into a basin of water, and then wipe it dry. Some of these girls have been known to suffer from lead poisoning.... Drop-wrist was frequently to be seen—in fact, there was hardly a workshop in the district in which cases of wrist-drop could not be found. They were all anæmic, and the albuminuric and prematurely aged were frequently met with.”
In this small industry in the past the poisoning must have been considerable. In 1898 nineteen cases were reported. Reference[285] to the table on p. 47 shows that the number now is greatly reduced. Those reported are generally cases which have ended fatally from the sequelæ of lead poisoning contracted many years previously.
Stained-glass painting—a form of vitreous enamelling—very rarely gives rise to poisoning, as no dust is generated (see vitreous enamelling for use of aerograph in glass-painting).
[33]—Most of the cases have occurred in the manufacture of white-lead paint, although manufacture of chromate of lead and of Brunswick greens (barytes with which Prussian blue and chrome yellows are mixed) account for several. The following table shows the precise occupation of persons affected, the number of cases distributed according to precise occupation, and the proportion of these to the total in 225 cases which were closely examined:
| Precise Occupation of Person affected. |
Number of Cases in each Subdivision. |
Proportion of Cases to Total (per Cent.). |
|---|---|---|
| Mixing and grinding (mainly of white lead) | 144 | 64·0 |
| Packing (mainly of red lead) | 19 | 8·4 |
| Sieving | 2 | 0·9 |
| Manufacture of chrome yellow | 22 | 9·8 |
| Colour house and filters | 16 | 7·2 |
| Painting and stencilling | 6 | 2·7 |
| Other processes | 16 | 7·0 |
Knowing the conditions of work, we can confidently assert that the poison must have entered the system in the form of dust in at least 90·0 per cent. of the cases, and in the remainder the possibility of dust having been the cause is not excluded.
In a small factory the cask of white lead is broken and the material scooped out into a pail. Scales are at hand, and when the amount of lead removed weighs half a hundredweight the contents of the pail are discharged either into a cylindrical pug-mill or into the pan of an edge-runner to be mixed with oil. In large factories the dry white lead is generally shovelled directly from the cask down openings or shoots in the floor to the grinding mills below.
—Dust arises in unheading the casks from the displacement of air following the scooping or shovelling out of the lead, in filling the pails, and in discharging the lead into the mill. All points should, and can, be adequately protected by locally applied exhaust ventilation at each one of the points enumerated. A telescopic arrangement of the branch duct in connection with the barrel enables dust generated in scooping out to be removed as the contents of the barrel get lower and lower (see Fig. 15).
[286]
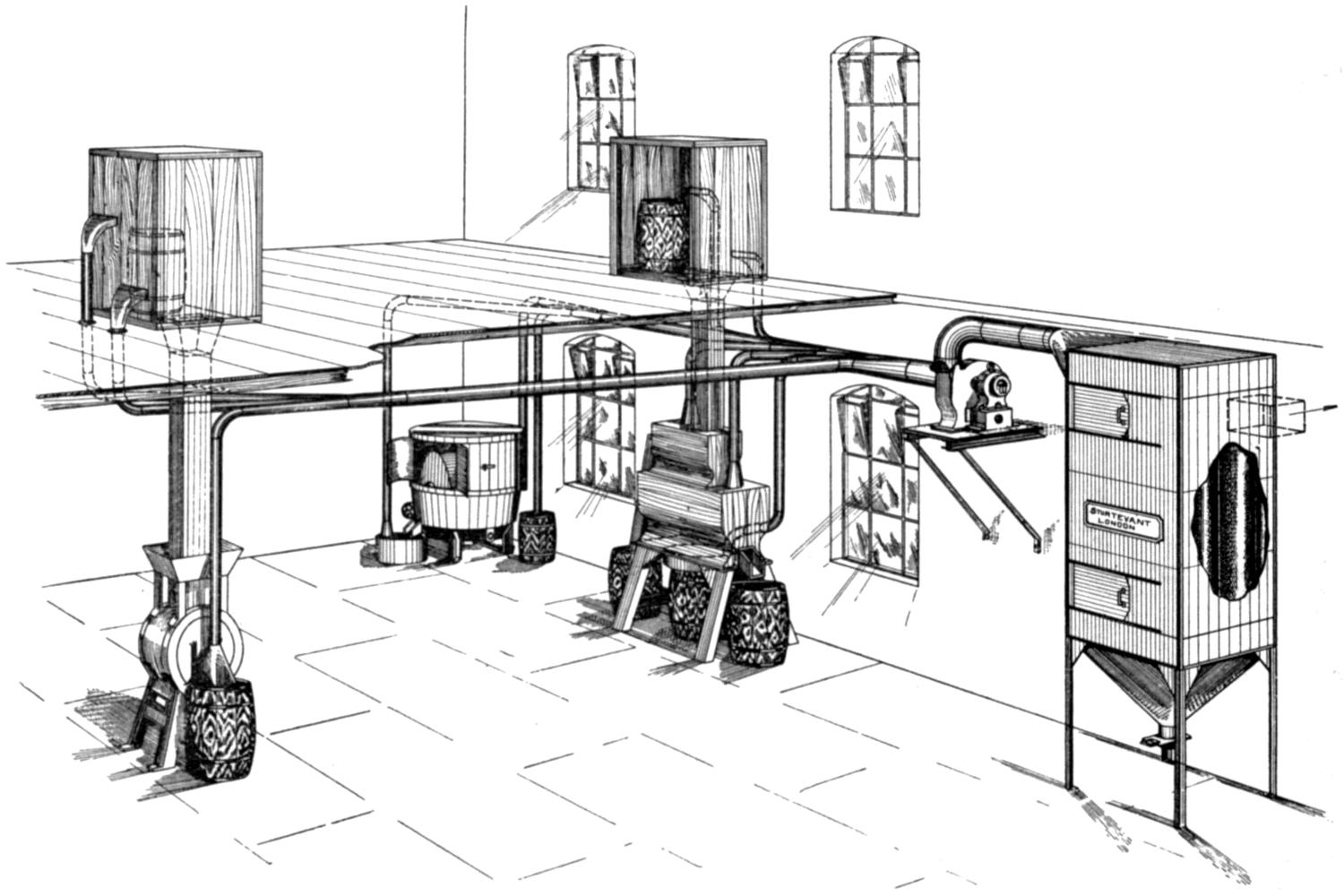
Fig. 15.[A]
[A] Fig. 15 shows the arrangement for preventing dust at every point where it is produced in a factory where dry colours are ground, sifted, and packed on a large scale. On the upper floor, the chamber is shown in which the contents of a cask are tipped down a shoot leading in the one case to the burr stone mill on the left, and in the other into the Blackstone sifters. Exhaust is arranged at two levels to catch the dust arising from the displacement of air. After grinding in the closed-in burr stone mill, a hood and duct is arranged over the point where the material is discharged into the barrel. Similarly, the casing of the two Blackstone sifters is connected with the exhaust fan, and also the cover of the barrel into which the ground material falls. Inside the edge-runner (the door of which is shown open) a negative pressure is maintained, and one branch duct controls the dust in the scooping out of the material from the barrel, while another is connected to the cover of the receptacle into which the ground material is discharged.
Tapering of the ducts, tangential entry of branches, fan-box, and collecting filters, are all shown. In the factory in question there are four edge-runners, three burr stone mills, and two Blackstone sifters. Altogether exhaust ventilation is applied at twenty-five points. (Drawing kindly supplied by the Sturtevant Engineering Company, Limited, London.)
[287]
The lighter shades of yellow chrome are made by a cold precipitation process, or (as is usual for the deeper shades of chrome, orange, and red) by boiling the ingredients—lead acetate, pulp white lead, bichromate of potash and soda, and sulphate of soda—while barytes is added as the colour is being made. Danger in the first method does not arise (or only in minor degree when steam is injected to bring about more speedy solution) until drying and grinding (in edge-runners), sieving, and packing, are effected. The dust, when inhaled, is quickly absorbed, and in all these dry processes danger, in the absence of very carefully thought out exhaust ventilation, is great. In processes involving ebullition, danger is present in the steam which carries up with it chromate of lead in fine particulate state. Vats and vessels, therefore, in which the boiling is effected require partial hooding over and connection of the hood with an efficient exhaust. In subsequent wet processes of pressing the cakes of chromate of lead, the hands, arms, and overalls become thickly coated with pigment. Danger from chrome greens is practically limited to the dust created in dry grinding, usually effected in large edge-runners.
For references, see end of Chapter XVII.
[288]
[34]—Lead poisoning is peculiarly prevalent in this industry, and no corresponding reduction in the number reported can be observed from year to year (see the table on p. 47), or in the many industries grouped under the heading, “Paint used in Other Industries,” such as is noted for lead industries taken as a whole.
Of the 697 cases included in returns during the ten years 1900-1909, 352 were reported from railway carriage and waggon works, 299 from ordinary carriage works and wheelwrights’ shops, and 46 (separate tabulation was only commenced in 1905) in motor-car works. In the year 1903 inquiry was made in 603 factories and workshops, including all classes of coach and carriage building, railway carriage and engine works, and agricultural implement works. Information was asked (among other things) as to—(1) The number of persons employed in painting with lead paints; (2) description of the method adopted for smoothing the coats of paint; and (3) the substitutes tried for white-lead paint. Persons employed numbered 9,608. In 52 factories and workshops smoothing of the coats of paint was not practised, while in the remaining 551 it was affirmed that a wet method alone (pumicestone and water) was used in 178, a dry method alone (sandpaper) in 39, and both wet and dry methods at some stage or other of the work in 334. Substitutes were mentioned as having been tried in 94 instances, but this was almost exclusively for filling and jointing, and not for the first or priming coat.
The figure 178 (wet method alone) is probably much too high, because, while it is true that pumicestone and water alone are used for the flat surfaces of the body—the bulk of the work—dry sandpapering of the first two priming coats and of the final[289] finishing coats (when of white, cream, or yellow colour), of the under parts of carriages, iron chassis of motor-cars, and of curved surfaces, such as the spokes of wheels, is almost universal. The reason for thus treating the priming coat dry is that a wet process would raise the grain of the wood. The 52 factories in which it was stated that no smoothing was done were nearly all premises for the repair or manufacture of railway trucks, requiring no special finish, and the 39 factories in which only sandpaper was said to be used in smoothing were premises in which rough, cheap, or common vehicles, such as carts, were made. Use of sandpaper is quicker and less expensive than use of pumicestone, and water and wet methods cannot be used very well on iron surfaces.
In ordinary coach and carriage painting, after the sandpapering of the first two priming coats, six or seven coats of “filling” (usually ground slate mixed with gold size and turpentine) are applied, and each coat is rubbed down wet. Joints and interstices of woodwork and irregularities in iron surfaces are generally filled in with a stopping or paste of white lead, in the smoothing of which sandpaper is used.
In the manufacture of motor-cars, the terne (lead) coated sheets which form the body, after preliminary preparation, receive two coats of a lead paint. These are either lightly sandpapered or “flatted” with pumice and water. Three coats of non-poisonous filling follow, and are flatted with pumice or German brick and water. The body then passes to a skilled workman, who applies the final coats of colour. For facing mouldings and for corners throughout all stages of the processes, dry sandpaper takes the place of pumice and water. All stopping on the chassis, the first lead coat on the bonnet, and all coats of paint on the wheels, are sandpapered dry. Sometimes a third of a man’s time may be taken up in sandpapering alone.
—Grave risk of inhaling lead dust is present (see the table on p. 47) when sandpaper is used, often at a point just above the mouth and nostrils. Rubbing down the wheels is perhaps the most dangerous work, and for this exhaust ventilation can be applied locally. Inventive genius has yet to be directed to some modification of the vacuum-cleaning apparatus, so that an exhaust can be attached to the back of the worker’s hand or in connection with a frame in which the sandpaper is held. In the process of wet rubbing, the abraded coats drip on[290] to the floor, and when dry may rise as dust into the atmosphere.
Precisely similar operations, or only modified in detail, have accounted for heavy incidence of lead poisoning in the painting of perambulators, of safes, of bicycles, of bedsteads, of gas-meters, the “metallic” enamelling of baths (in which also chipping off of the old paint not infrequently occasions an attack), in engineering and machine-making works, in cabinet and furniture making, in French polishing, in the making of artists’ canvases, etc. Several cases are reported among railway employees engaged in the painting of bridges, girders, and signal-posts. A method for the removal of the dust given off in these processes has not yet been arranged. Chipping off of old paint can be effectually replaced by solvent solutions, in the use of which, as they are very inflammable, precautions against naked lights are necessary.
In the making of better-class measuring tapes, the tape, after passage through the white-lead mixture and drying, is made to travel through a machine to remove roughnesses, and subsequently through the fingers of the worker, protected by leather. Dust arises in both the last operations, and requires to be removed by exhaust ventilation. Similar means of prevention are necessary wherever paint is applied, as in photo-engraving, and colouring artificial flowers by means of an aerograph instrument.
Owing to the limited extent to which exhaust ventilation is possible, reliance must be placed on substitution of wet processes for dry wherever possible. Cleanliness of floors requires special attention. Although in all painting operations dust is the most potent cause of poisoning, we would assign to contamination of the hands and the eating of food with unwashed hands a more prominent place as a cause than in any of the other processes involving use of lead or lead colours. In a post-mortem on a sign-painter employed only a few days, made three weeks after his cessation of employment on account of an attack of encephalopathy, paint was found thickly adherent under the nails.
Substitution of colours containing no lead suggests itself as a simple remedy, but the progress in this direction made so far in the industries mentioned is limited. Several important firms manufacturing motor-cars use no lead colours at all; more than one important railway company (the outside of the carriages of[291] which has no white colour) and a few makers of perambulators do the same. It is difficult to obtain knowledge how far leadless are replacing lead colours. In the manufacture of cornice poles (in which small industry several severe attacks were reported) the suggestion of a factory inspector to employ lithopone was adopted, with entire success. A patent graphite has been substituted for orange lead, with which wooden patterns to form the moulds of articles to be subsequently cast in metal are frequently painted.
[35]—The work of house-painting and plumbing outside a workshop does not come under the Factory and Workshop Act, 1901, except to a limited extent under Section 105 in buildings in course of erection; and even in that case the requirement of notification of lead poisoning imposed by Section 73 does not apply. If, however, a house-painter is employed for part of his time in mixing paints in a workshop belonging to a builder, then the question may legitimately be raised as to whether plumbism may not have been due in some measure to such workshop conditions. Despite the limited extent to which the Act applies to lead poisoning of house-painters and plumbers, seeing that it is industrial in origin many practitioners notify cases, with the result that the number every year exceeds considerably that from any other lead industry in the country. Thus, the number notified in the ten years 1900-1909 was 1,973, including 383 deaths. The proportion of deaths to persons notified is much higher than for lead industries generally (19·4 per cent., as compared with 4·0 per cent.). If the proportion of cases to deaths were the same in house-painting as in other industries (and it is a fair assumption to make), the number of cases would be 9,418.
When investigation is made into the reported cases, the pre-dominance of the severer symptoms—paralysis, brain symptoms, and chronic plumbism—is brought out. Causation of poisoning, in order of importance, appears to be: (1) Dust from sandpapering one surface of paint before applying another; (2) dust from mixing dry white lead with oil; (3) dust arising from paint that has dried on overalls; (4) contamination of food with unwashed hands; and (5) fumes from burning off old paint.
—Opinion still differs as to the feasibility of substituting zinc sulphide or zinc oxide (or a combination of the two) for white lead in paints, in spite of elaborate investigation of the point by commissions of inquiry appointed[292] notably by the French, Austrian, and Dutch Governments. There is, however, general consensus of opinion that for the painting of internal surfaces of houses and of all surfaces which are not exposed to the weather zinc paints have the advantage (apart from their non-poisonous quality) over white-lead paint of not changing colour. The technique for applying zinc oxide paint differs much from that for applying white lead. Being much less dense, it requires to be ground with a greater proportion of oil, and the vehicles and driers necessary for the thinning of the stiff paste are different from those ordinarily used for thinning and mixing white lead. Coats of zinc oxide should be applied as thin as possible, and hence there is the drawback that where three coats of white lead will suffice, four coats of zinc oxide may be necessary unless the paint is skilfully applied. The best method of applying zinc oxide paint with the brush has to be learnt in order to get the best effect. The ordinary house-painter, therefore, accustomed to the use of lead paint, cannot expect to obtain the same result from zinc paint treated in the same way. And zinc oxides differ in value as pigments according to the methods of production. That obtained by direct roasting of the ore (franklinite and zincite) is superior to that prepared by the indirect method of oxidation of spelter.
Zinc sulphide enters into the composition of many white paints mixed with zinc oxide, barytes, and often lead sulphate. Its defect in colour is thus concealed, and it adds to the mixture the important property known to the painter as “body.” Under a variety of names, such as “Orr’s enamel white,” “patent zinc white,” and “lithopone,” such mixtures have a large sale, and for many purposes can act as a substitute for white-lead paint.
Extensive inquiries have been made in recent years in Continental countries into the effect of use of white-lead paint in producing plumbism, the processes employed, and the possibility of substitutes—in Austria, from 1904 to 1907; in Germany, in 1905; in Holland, from 1903 to 1909; in France, from 1901 to 1909; in Switzerland, in 1904; and in Belgium, from 1904 to 1909. In 1902 the French Government, by a decree applying to house-painting, prohibited (1) use of white lead except when ready mixed with oil; (2) direct handling of white lead; (3) dry-rubbing or sand-papering of painted surfaces; and required (4) provision of the usual means for cleanliness, including overalls. This[293] decree in 1904 was extended to all kinds of painting with use of white lead. Finally, in 1909, a law, to take effect from 1914, was passed prohibiting the use of white lead in paint altogether.
In Belgium, following on regulations issued under royal decree in 1905, in which, among other things, quarterly periodical medical examination of house-painters was required, the law dated August 20, 1909, came into force, prohibiting the sale, transport, and use, of white lead in the form of powder, lumps, or small pieces, and requiring, if intended for the purpose of painting, the white lead to be mixed ready ground in oil. Dry-rubbing and sandpapering are also prohibited.
In the German Empire the work of house-painting is controlled by regulations dated June 27, 1905, of which the following are the main provisions: (1) Prohibition of actual contact with white lead in grinding and mixing, and adequate protection from the dust so created; (2) mechanical incorporation of the white lead with the oil or varnish, and prevention of the escape of dust into the workroom; (3) preliminary moistening prior to scraping, chipping off, or rubbing down, dry oil colours; (4) and (5) provision of overalls and washing accommodation, including soap, nailbrushes, and towels (in erection of new buildings the workmen must be able to wash in a place free from frost); (7) instruction of the workman by the employer as to the risk attaching to the work by supplying him with a copy of the regulations and cautionary notice. Further, where painting operations are carried on in factories or workshops as subsidiary to other processes, there must be (8) provision of washing accommodation in a special room capable of being heated, and of a place in which to keep clothing; (9) periodical medical examination at half-yearly periods; and (10) prohibition of smoking and consumption of alcohol in the workrooms.
The Austrian Regulations, dated April 15, 1909, follow the German Code closely, but differ in that they (1) prohibit the use of white lead paint for the interior surfaces of houses or of any surfaces not exposed to the weather; (2) affixing of a notice on the can or cask that it contains lead; and (3) periodical medical examination at quarterly instead of half-yearly periods.
At the present time committees appointed by the Home Office are inquiring into the coach-painting and house-painting industries in this country.
The results of careful and detailed experiments made by the[294] White Lead Commission appointed by the Dutch Government, which inquired into the subject, are summarized as follows:
I. Zinc-white paints are much better able to withstand the action of sulphuretted hydrogen gas than white-lead paints.
II. Zinc-white paints do not withstand the action of sulphurous acid in the atmosphere as well as white-lead paints.
As this gas is present in coal-smoke of locomotives, steamers, tall chimneys, etc., zinc-white paint much exposed to such smoke—for instance, in railway-stations, etc.—will soon become corroded, and cannot then replace white lead.
III. Zinc-white paints applied on zinc, Portland cement, or iron (the latter having previously been provided with first coats of red oxide of lead or iron), are able to withstand the action of the open air for a space of five years quite as well as white-lead paints, and can entirely replace the latter, provided they are not exposed to the action of vapours containing sulphurous acid.
IV. In the interior of buildings zinc-white paints, applied on wood, iron, zinc, Portland cement, and plaster, are as good as white-lead paints; and can entirely replace the latter, provided they are not exposed much to vapours containing sulphurous acid or to much damp.
V. Zinc-white paints applied on wood, if not exposed much to the action of sulphurous acid gas, will in many cases last during five years in the open air as well as white-lead paints, and can replace the latter with good results. But in all places where water accumulates, as on window-sills, the lower side of cornice-work, etc., they will, even after three or four years, deteriorate to such a degree that repainting will become necessary for the preservation of the wood; in this respect, therefore, they are inferior to white-lead paints.
VI. Zinc-white paints, such as the White Lead Commission have used successfully, cover at least equally as well as the white-lead paints customary in this country.
The zinc-white putty used by the White Lead Commission is quite as serviceable as ordinary white-lead putty.
VII. Painting with zinc-white paint, such as the Commission used on new woodwork in the open air, does not cost more than painting with the white-lead paints customary for that purpose.
VIII. Painting on existing paintwork, so-called “repainting,” in the open air, with zinc-white paints such as the White Lead[295] Commission used, costs more than the white-lead paints hitherto in use, inasmuch as the preparation of the wood painted with zinc-white paints involves greater expense in rendering it fit for the repainting than in the case of wood painted with white lead in rendering it fit for further painting with white lead.
In the case of painted wood which is exposed to the open air, the possibility is, moreover, not excluded that, where such wood is in an unfavourable condition of humidity (see under § V.), it may have to be repainted sooner than if it had been painted with white-lead paints.
In these circumstances the cost of maintenance of wood painted with zinc-white paint, and exposed to the open air, is further increased as compared with wood painted with white-lead paint.
IX. Lithopone paints cannot replace white-lead paints in the open air; they have proved to be quite unfit in this respect.
X. For paintwork above water, first coats of oxide of iron have, during five years, proved to be quite as good and serviceable as first coats of red oxide of lead.
For coats of paint under water, oxide of iron cannot be used.
Coats of oxide of iron paint are cheaper than coats of red oxide of lead paint.
When oxide of iron is used for the first coat, much more technical skill is required for the painting of the covering coats than is the case when red oxide of lead is used for the first coat.
[36]—Cases arising in shipbuilding are due not so much to mixing the paints or red-lead paste as to the dust produced in sandpapering the coats of white paint applied in cabins, etc., in chipping and scraping off old red-lead paint, often in confined spaces such as double bottoms, tanks, bilges, etc. Splashing from injecting red lead between plates, fumes from burning off old paint, and fumes from paint while using it in confined spaces, are mentioned in reports. Several attacks have occurred to persons engaged in inserting red-hot rivets into holes containing yarn soaked in red lead and oil. Lead fumes, it is suggested, are given off. The number of cases included under this heading each year has been—
| 1900 | 32 |
| 1901 | 28 |
| 1902 | 15 |
| 1903 | 24 |
| 1904 | 48 |
| 1905 | 32 |
| 1906 | 26 |
| 1907 | 22 |
| 1908 | 15 |
| 1909 | 27 |
| 1910 | 21 |
The figures illustrate the difficulty of obtaining a reduction in[296] the attacks when the cause is to be found in conditions not amenable to control by exhaust ventilation. The possibility of effecting some reduction by such precautions as can be adopted is suggested by the diminution (from 110 to 60) in the number of cases in the Government dockyards in the six years 1905-1910 and 1899-1904 respectively, as compared with the increase (from 67 to 87) in all other shipbuilding yards.
In the Government dockyards, among other precautions, men employed on red-leading appear before the medical officer periodically, and no man is allowed to do the work for more than two days a week. Further, oxide of iron paint is to be used in the double bottoms, wing passages, and other confined spaces on board ships. All men employed as painters are allowed five minutes out of their working time for washing.
—The industries and processes which are gathered together under this head will be seen from the following distribution:
| Industries. | Cases (Ten Years: 1900-1909). |
|---|---|
| (1) Iron drums and kegs | 47 |
| (2) Harness furniture | 23 |
| (3) Tempering springs | 13 |
| (4) Other contact with molten lead | 103 |
| (5) Metal sorting | 13 |
| (6) Handling lead and dust from metallic lead | 122 |
| (7) Shot-making | 14 |
| (8) Glass-making | 13 |
| (9) India-rubber | 23 |
| (10) Yarn-dyeing | 28 |
| (11) Copper letters and opal signs | 28 |
| (12) Other lead compounds | 196 |
| (13) Miscellaneous | 36 |
| Total | 659 |
(1) and (2) have been described under tinning of metals, as the processes are similar, and in the year 1909 they were included along with tinning of hollow-ware under the same code of regulations.
Tempering of steel buffer springs (3)[37], carried on in Sheffield, gives rise to poisoning from fumes of molten metal into which the springs are immersed, and from dust of skimmings, unless there is efficient hooding and exhaust. A sample of dust collected from a lampshade over a melting-pot was found in the Government laboratory to contain 48·1 of metallic lead, or 51·8 per cent. of lead monoxide. In testing the springs under a hydraulic press, and subsequent straightening by hammering on[297] an anvil, the thin coating of lead on the surface scales off, and may be inhaled.
Other contact with molten metal (4) includes operations which do not differ from several already described, in which danger is incurred from either fumes and dust in skimming the dross or subsequent handling, such as manufacture of solder, coating cables, filling copper cylinders with molten lead for the purpose of bending them, and subsequently re-immersing them in the bath to melt out the lead, tinning of nails, making lead patterns for fenders (in which there may be danger, also, from use of a wire brush to get rid of adhering sand), etc.
Handling lead and dust from metallic lead (5) includes operations such as die-stamping, stamping tickets and other articles on a leaden slab (where the danger is akin to, though probably less in degree than in file-cutting), examining bullets, manufacture of metallic capsules, lining boxes with sheet lead, lead glazing (where the danger is essentially that of plumbing work), etc.
It includes also a number of cases which were reported previous to 1905 in the markers of testing ranges at a small-arms factory. Duckering[38], who investigated these cases, found that the bullets were stopped by dry sand in boxes 8 feet long. On entering the sand the bullets became disintegrated, so that, after being in use for some time, the sand contained a large amount of lead, and had to be removed. In doing this the box was turned over, and the sand deposited on the floor immediately behind the targets. The lead was then separated by sifting by hand, and the sand used over again. In these operations much floating dust was produced, which was inhaled by the markers, who stood in an open trench immediately in front of and below the targets.
—Some cases have occurred from the manufacture of capsules for bottles. The capsule consists of a lead leaf rolled between two leaves of tin. Cases arising in the early processes of casting and rolling do not differ from those described as due to contact with molten metal and handling of lead. The most difficult to deal with are those which occur in the final process of cleaning and colouring. Before colouring with varnish paint, the capsule is placed on a rapidly revolving lathe, and the hand of the worker, carrying a cloth containing whitening, is placed lightly on the capsule. A slight amount of dust is inevitably raised, and this dust, collected from the bench, was found[298] to contain from 11·5 to 25·6 per cent. of lead; while dust which had settled on a beam 9 feet from the floor contained 9·3 per cent. Of thirty-one workers employed in cleaning and colouring, fifteen showed evidence of lead absorption in a blue line on the gums, and in one there was considerable weakness of the left wrist. Similar experience of lead poisoning in this industry has been noted in German and Austrian factories.
Periodical medical examination at quarterly intervals has been instituted in the principal factory, with good results, as it enables those who show early signs of lead absorption to be transferred to other processes. Exhaust ventilation has been tried, but, except at the few lathes where cleaning alone is done, without complete success, in view of the nature of the work.
—Cases in shot-making arise from the dust given off when sifting the shot into different sizes—an operation which should be carried on in sieves entirely closed in and under negative pressure. Dust collected from the glass casing over a sifting machine contained 60·3 per cent. of metallic lead. The sample was free from arsenic.
—Cotton yarn is dyed (10) on a considerable scale with chromate of lead, chiefly for Oriental markets; and it is the orange chrome—that most heavily weighted with lead—which is most in demand there. The orange chrome colour is obtained by dipping hanks of yarn into solution of lime, and then into acetate of lead. The process is repeated a second time, after which the chromate is formed by dipping in bichromate of soda, and finally boiling in lime-water[39].
In production of yellow chrome colour, the yarn is treated only once in a bath of lead acetate. Other colours made are lemon chrome and (by addition of an indigo bath) chrome green.
The early processes of dyeing rarely give rise to poisoning, but the strong solution of bichromate of soda readily causes characteristic ulceration of the skin—“chrome holes.” Danger arises from dust in the process of heading or “noddling,” as it is sometimes called, of the dried yarn over posts. The hanks of yarn are tugged and shaken by women as a rule, and in the case of orange chrome very considerable quantities of dust are liberated. We have been told that a hank of this kind of yarn does not commend itself to an Oriental buyer unless, when shaken, dust is visible.
[299]
The industry was certified as dangerous in 1895, in view of serious illness and death in Glasgow and Manchester, and special rules were made to apply, not only to the heading operations, but also to the winding, reeling, and weaving, of the dyed yarn—processes in which cases of poisoning are very rare.
Detailed inquiry was made in 1906 in eleven factories where yarn was dyed on a considerable scale by means of chromate of lead—in eight mainly for export to India, and in three for the home market. Yarn dyed for the home market gives off less dust when headed, as the material undergoes additional washing in water and in dilute acid; and it is also sometimes passed through a sizing of starch, which fixes the chromate of lead to the yarn more securely.
Proof of the greater danger from orange chrome is found in the fact that Dupré was able to wash 1 pound of dust (0·29 per cent.) from 345 pounds of heavy orange yarn, and only 1 pound (0·03 per cent.) could be washed from 3,300 pounds of light yellow or green yarn.
In none of the factories were the workers engaged solely on the dangerous yellow and orange chrome-dyed yarn. In some the work may last an hour or two every day, in others for an hour or two every day in alternate weeks, or for one week in every three or four weeks, and perhaps in a dozen factories the work may not be done more frequently than half a day a month, or even one in three months.
Particular attention was paid to the nature of the exhaust ventilation at the “heading” posts, as this is the most important point in the protection of the workers. It was provided in eight out of the nine principal yarn-dyeing factories. The exception was one where the work was said to be solely for the home market. In one a 2 foot 6 inch Blackman fan was placed in the wall without connection of the “heading” posts with it by means of ducts and hoods. In four, hoods and ducts of wood, square in section, with right-angle bends, had been locally applied to the posts. In other four, hoods and ducts were of metal, circular in section. The velocities in feet per minute (obtained with a Davis self-timing anemometer) were taken at the opening into the branch duct behind or under the post. The value of anemometric tests in detecting blockages or interference in the ducts is evident from the table on p. 300.
[300]
| (1) | (2) | (3) | (5) | (6) | (7) | (8) | (10) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fan. | Fan. | Fan. | Fan. | Fan. | Fan. | Fan. | Fan. | Fan. | Fan. | Fan. | Fan. |
| 240 | 820 | 330 | Nil | 1,200 | 420 | 450 | 210 | 780 | 570 | 700 | 850 |
| 450 | 450 | 20 | 420 | 510 | 210 | 570 | 700 | ||||
| 480 | 270 | 450 | 270 | 780 | 360 | 420 | 390 | 660 | 540 | 490 | 850 |
| 480 | (750) | 420 | 270 | 360 | 420 | 430 | 540 | 570 | 570 | ||
| 480 | 330 | Nil | 250 | 270 | 120 | 420 | 510 | 540 | 530 | ||
| 450 | (440) | Nil | 300 | 300 | 120 | 490 | 540 | 540 | |||
| 324 | 320 | 300 | 180 | 350 | 480 | 450 | 300 | 300 | 450 | ||
| 280 | (420) | 250 | 150 | 290 | 480 | 420 | 300 | 450 | |||
| 25 | 130 | 350 | 430 | 390 | 510 | 420 | |||||
| 25 | 220 | 180 | 420 | 360 | 460 | 400 | |||||
| 360 | 300 | 240 | 420 | ||||||||
| 240 | 280 | 450 | 480 | ||||||||
| Nil | 210 | 390 | |||||||||
| Nil | 210 | 390 | |||||||||
| Nil | |||||||||||
| Nil | |||||||||||
(1) The draught here was obtained from the main chimney-shaft. The small velocities at the end post, it was subsequently found, arose from the fact that the double heading post was connected by means of a very small duct to the end of the large duct which served the other posts.
(2) Wooden duct connected up with fan. The area of the openings into the duct could be enlarged or diminished by means of a shutter. The figures in brackets were those obtained when the shutter was fully opened.
(3) In this factory originally a 2 foot 6 inch fan was simply placed in the wall. Subsequently they were boxed in and ducts of wood brought within a foot of the noddling bar. Four of the branch ducts were found to be blocked.
(5) Wooden ducts and hoods behind bar both close to the fan.
(6) Circular metal ducts with curved angles, and placed about 8 to 10 inches behind post; all connected up with a 4 foot 6 inch fan. The small velocities (120 feet) at two posts was due to loose connection of the branch ducts allowing air to be drawn in at the foot.
(7) Metal duct distant about 2¹⁄₂ feet from the post, and situated immediately below and not behind the bar. Dust was prevented from rising above the post by a glass screen, the projection[301] of which also prevented the worker from coming too near to, or getting his head over, the post.
(8) Metal ducts, 9¹⁄₂ inches in diameter. Evidence of ill-health was greatest here, notwithstanding good draught, because the branch ducts were not brought close enough to the point where “heading” was done, but were distant 15 inches from the centre of the post, and “noddling” was done at a distance of 2 feet from the duct, one man standing between the draught and the bar.
(10) Draught arranged as in (7), below the bar, without protection of the worker by a glass screen.
Regulations now apply to the industry. So clear is it that locally-applied exhaust ventilation is of paramount importance in prevention of poisoning, that, however intermittent the operation of “heading,” exemption from this requirement cannot be permitted. Determination periodically by the occupier of the speed of the draught at each exhaust opening should prevent blockage of ducts.
The regulations do not apply to the winding of, and weaving with, yarn dyed with chromate of lead. Rarely in the spinning and weaving factories of Blackburn does the amount of the particular yarn in question constitute as much as 5 per cent. of the total quantity of coloured yarn used. Section 74, 1901, is sufficient to meet the isolated cases where injury to health arises. The habit of biting chrome-dyed thread has given rise to lead poisoning. Nor do the regulations apply to treatment of calico or cloth into which lead may enter. Such poisoning as may occur must be practically confined to persons employed in the paint-mixing house.
[9]—Litharge, massicot, red lead, and sulphide of lead, are generally mixed with rubber. Litharge is regarded not only as a valuable filler for rubber, but has the faculty of hastening vulcanization. All dry-heat goods depend upon it where a dark or black effect is wanted.
Every year a few cases are reported in the process of mixing the batches in the weighing room of the rubber factory, or more frequently at the hot calender rolls, where the batch of dry powder containing the lead compound is gradually distributed by hand on to the rubber so as to effect an intimate mixture. The heated air over the rollers causes dust to rise. According to the purpose for which the rubber is wanted, the quantity of litharge in the batch varies. In one factory of fourteen men employed at the calender rolls, ten showed a blue line, five were[302] markedly anæmic, one had weakness of the wrists, and two weakness of grasp[40]. Only one case has been reported since exhaust ventilation was applied locally over each calender roll. In a rubber tyre factory five cases followed one another in quick succession, all in persons employed on the rolls. There should be no hesitation in requiring exhaust ventilation wherever employment in mixing the batches or at the rolls is constant. In general, however, the work in weighing out is intermittent, and reliance is placed on the wearing of a respirator.
No attempt has been made to enumerate all the industries and processes in which lead poisoning may arise. The task would become wearisome, as they are so numerous. Nor is it necessary to give details of all that are known, as it is doubtful whether there can be any different in nature or requiring different treatment from the many which have been described.
[1] Special Report on Dangerous or Injurious Processes in the Smelting of Materials containing Lead, and in the Manufacture of Red and Orange Litharge and Flaked Litharge, by E. L. Collis, M.B. Cd. 5152. 1910. Wyman and Sons, Ltd. Price 6d.
[2] Annual Report of the Chief Inspector of Factories for 1901, p. 213.
[3] Ibid., p. 242.
[4] Ibid. for 1906, p. 272.
[5] H. O. Hofman: Metallurgy of Lead. 1906.
[6] Annual Report of the Chief Inspector of Factories for 1900, p. 438.
[7] Ibid. for 1910, p. 154.
[8] Special Report above, p. 15.
[9] Layet: Quoted by Oliver in Dangerous Trades, p. 288.
[10] Dixon Mann: Forensic Medicine and Toxicology, p. 477.
[11] Sommerfeld: Bekämpfung der Bleigefahr, p. 220.
[12] Sommerfeld: Quoted by Silberstein below, p. 257.
[13] Silberstein: Die Krankheiten der Buchdrucker, in Weyl’s Handbuch der Arbeiterkrankheiten, p. 257. Gustav Fischer, Jena, 1908.
[14] Tatham: Decennial Supplement to Sixty-fifth Annual Report of the Registrar-General. Cd. 2619.
[15] Third Interim Report of the Departmental Committee on Certain Miscellaneous Dangerous Trades. C. 9073. 1898.
Report on the Draft Regulations for File-Cutting by Hand, by Chester Jones. Cd. 1658. 1903.
[16] Annual Report of the Chief Inspector of Factories for 1904, p. 125.
[17] Ibid. for 1906, p. 273.
[18] Special Report on Dangerous or Injurious Processes in the Coating of Metal with Lead or a Mixture of Lead and Tin, by Miss A. M. Anderson, H.M. Principal Lady Inspector of Factories, and T. M. Legge, M.D., H.M. Medical Inspector of Factories; together with a Report on an Experimental Investigation into the Conditions of Work in Tinning Workshops, and Appendices, by G. Elmhirst Duckering, one of H.M. Inspectors of Factories. Cd. 3793. London: Wyman and Sons, 1907.
Annual Report of the Chief Inspector of Factories for 1902, pp. 296-318.
Report on Draft Regulations on the Tinning of Metal Articles, by E. T. H. Lawes.
The Cause of Lead Poisoning in the Tinning of Metals, by G. E. Duckering.
[303]
[19] The Health of Brass Workers, by T. M. Legge. Annual Report of the Chief Inspector of Factories for 1905, pp. 388-397.
[20] Ibid. for 1898, pp. 119-123; and many references in later Annual Reports.
[21] The Bischof Process for the Manufacture of White Lead, by Professor Sir William Ramsay, K.C.B., D.Sc. 1906.
[22] Report of the Departmental Committee on the Use of Lead, and the Danger or Injury to Health arising from Dust and Other Causes in the Manufacture of Earthenware and China: vol. i., Report; vol. ii., Appendices. Cd. 5277-8. 1910.
Lead Compounds in Pottery: Report to H.M. Principal Secretary of State for the Home Department on the Employment of Compounds of Lead in the Manufacture of Pottery; their Influence upon the Health of the Workpeople; with Suggestions as to the Means which might be adopted to Counteract their Evil Effects, by Professor T. E. Thorpe, LL.D., F.R.S., Principal of the Government Laboratory; and Professor Thomas Oliver, M.D., F.R.C.P., Physician to the Royal Infirmary, Newcastle-on-Tyne. London: Eyre and Spottiswoode, February, 1899. Price 5¹⁄₂d.
[23] Work of the Government Laboratory on the Question of the Employment of Lead Compounds in Pottery, by Professor T. E. Thorpe. Cd. 679. 1901.
[24] H. R. Rogers: Report of a Series of Experiments for Determining the Amount of Lead in the Glaze of Finished Ware, based on the Method described by Sir Henry Cunynghame, K.C.B., in his evidence before the Departmental Committee on the Use of Lead (see 22, above).
[25] See 22, above, pp. 93, 94.
[26] C. R. Pendock: Unpublished Report.
[27] Special Report on Dangerous and Injurious Processes in the Enamelling and Tinning of Metals, by Miss A. M. Anderson and T. M. Legge, in Annual Report of the Chief Inspector of Factories for 1902, pp. 296-318.
[28] Annual Report of the Chief Inspector of Factories for 1910, p. 154.
[29] Zeitschrift für Gewerbehygiene, Unfall Verhütung und Arbeiter-Wohlfahrtseinrichtungen, January, 1902.
[30] Annual Report of the Chief Inspector of Factories for 1901, pp. 221-229.
Die in electrischen Akkumulatoren Fabriken beobachteten Gesundheitsschädigungen. Arbeiten aus dem Kaiserlichen Gesundheitsamte, by Dr. Wutzdorff. 1898.
[31] Third Interim Report of the Departmental Committee appointed to inquire into and report upon Certain Miscellaneous Dangerous Trades, pp. 16-19. C. 9073. 1898.
[32] D’Arcy Ellis: Brit. Med. Journ., vol. ii., pp. 406-408, 1901.
[33] Report on the Manufacture of Paints and Colours containing Lead, as affecting the Health of the Operatives employed, by T. M. Legge, M.D. Cd. 2466. 1905.
Painters’ Colours, Oils, and Varnishes, by G. H. Hunt, Griffin, p. 357. 1901.
[34] Annual Report of the Chief Inspector of Factories for 1905, pp. 366-368, and references in other Annual Reports.
[35] Report of the Departmental Committee appointed to inquire into the Dangers attendant on Building Operations, Appendix IX., pp. 184-187. Cd. 3848. 1907.
Painters’ Colours, Oils, and Varnishes, by G. H. Hunt, Griffin. 1901.
[36] Annual Report of the Chief Inspector of Factories for 1910, pp. 175-176.
[37] Ibid. for 1906, pp. 272, 273.
[38] Annual Report of the Chief Inspector of Factories for 1905, pp. 368, 369.
[39] Dangerous Trades Committee’s Final Report, C. 9509, pp. 26-30.
Alex. Scott: Minutes of Evidence of Various Lead Industries Committee, 1894, C. 7239-1, pp. 105-108.
J. S. Clayton: Industrial Lead Poisoning among Yarn Workers. Brit. Med. Journ., vol. i., p. 310, 1906.
[40] Annual Report of the Chief Inspector of Factories for 1901, p. 231.
BILLING AND SONS, LTD., PRINTERS, GUILDFORD
The text used in this document is the one used in the source document, except as mentioned below. In particular, non-English words and phrases and titles and author names of references, and summations and other calculations in tables have not been corrected, unless mentioned below. Table numbering (or the lack thereof) has not been standardised.
Depending on the hard- and software used to read this text and their settings, not all elements may display as intended.
Page 1, 6: Stockhusen is probably an error for Stockhausen.
Page 38, the reference to Goadby[13] points to a publication by Garrod.
Page 52: hyperthenar eminence may be an error for hypothenar eminence.
Page 64, ... se recontrant d’une manière diffuse ...: possibly an error for ... se rencontrant d’une manière diffuse ....
Page 70, bottom row of Table IX: possibly the first column should read Average age at death.
Page 220, Literature reference [5]: there is no footnote marker in the text, and therefore no back-link to the text.
Changes made
Tables and illustrations have been moved out of text paragraphs. Some of the tables have been re-arranged or split for legibility; ditto marks and the word Ditto have in several places been replaced with the dittoed text.
Some obvious minor typographical and punctuation errors have been corrected silently.
Page 3: ... mascicot and litharge ... changed to ... massicot and litharge ....
Page 47, Table III bottom part: value for 1911 in the row Other industries changed from 8 to 88⁴ (based on the totals given).
Page 61, Footnote [2]: Dr. John Tathan changed to Dr. John Tatham.
Page 75: Pfleuger changed to Pflueger.
Page 76: Seiffert changed to Seifert.
Page 79, references [16] and [27]: Bleilähnung changed to Bleilähmung; reference [49]: les Maladies du Pois et Reins changed to les Maladies du Foie et des Reins.
Page 88: eutetic entangling changed to eutectic entangling.
Page 151: Electrical Reactions. changed to Electrical Reactions.
Page 169: magnese changed to manganese.
Page 220: Leclere de Pulligny changed to Leclerc de Pulligny.